Efficacy and safety of upadacitinib for active ankylosing spondylitis refractory to biological therapy: a double-blind, randomised, placebo-controlled phase 3 trial
- PMID: 35788492
- PMCID: PMC9606523
- DOI: 10.1136/ard-2022-222608
Efficacy and safety of upadacitinib for active ankylosing spondylitis refractory to biological therapy: a double-blind, randomised, placebo-controlled phase 3 trial
Abstract
Objectives: To evaluate the efficacy and safety of upadacitinib, a Janus kinase inhibitor, in patients with active ankylosing spondylitis (AS) with an inadequate response (IR) to biological disease-modifying antirheumatic drugs (bDMARDs).
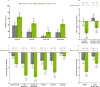
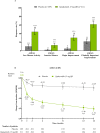
Methods: Adults with active AS who met modified New York criteria and had an IR to one or two bDMARDs (tumour necrosis factor or interleukin-17 inhibitors) were randomised 1:1 to oral upadacitinib 15 mg once daily or placebo. The primary endpoint was Assessment of SpondyloArthritis international Society 40 (ASAS40) response at week 14. Sequentially tested secondary endpoints included Ankylosing Spondylitis Disease Activity score, Spondyloarthritis Research Consortium of Canada MRI spine inflammation score, total back pain, nocturnal back pain, Bath Ankylosing Spondylitis Functional Index, Bath Ankylosing Spondylitis Metrology Index and Maastricht Ankylosing Spondylitis Enthesitis Score. Results are reported from the 14-week double-blind treatment period.
Results: A total of 420 patients with active AS were randomised (upadacitinib 15 mg, n=211; placebo, n=209). Significantly more patients achieved the primary endpoint of ASAS40 at week 14 with upadacitinib vs placebo (45% vs 18%; p<0.0001). Statistically significant improvements were observed with upadacitinib vs placebo for all multiplicity-controlled secondary endpoints (p<0.0001). Adverse events were reported for 41% of upadacitinib-treated and 37% of placebo-treated patients through week 14. No events of malignancy, major adverse cardiovascular events, venous thromboembolism or deaths were reported with upadacitinib.
Conclusion: Upadacitinib 15 mg was significantly more effective than placebo over 14 weeks of treatment in bDMARD-IR patients with active AS. No new safety risks were identified with upadacitinib.
Trial registration number: NCT04169373.
Keywords: Magnetic Resonance Imaging; antirheumatic agents; inflammation; spondylitis, ankylosing.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DvdH has received consulting fees from AbbVie, Bayer, BMS, Cyxone, Eisai, Galapagos, Gilead, GSK, Janssen, Lilly, Novartis, Pfizer, and UCB Pharma; is the director of Imaging Rheumatology BV; is an Associate Editor of the Annals of Rheumatic Diseases; is an editorial board member of the Journal of Rheumatology; and is an advisor for the Assessment in SpondyloArthritis international Society. XB has received grant/research support from AbbVie and Novartis; consulting fees from AbbVie, BMS, Chugai, MSD, Novartis, Pfizer, and UCB; speakers’ bureau fees from AbbVie, BMS, Celgene, Chugai, Merck, Novartis, Pfizer, and UCB; and is an editorial board member of the Annals of Rheumatic Diseases. JS has received grant/research support from AbbVie, Merck, and UCB; has been a consultant for AbbVie, Merck, Novartis, and UCB; and has served on the speakers’ bureau for AbbVie, Merck, and Novartis. AD has received grant/research support from AbbVie, BMS, Celgene, GSK, Lilly, Novartis, Pfizer, and UCB; and honoraria or consultation fees from AbbVie, Amgen, Aurinia, BMS, Celgene, GSK, Janssen, Lilly, MoonLake, Novartis, Pfizer, and UCB. RDI has received grant/research support from AbbVie, Amgen, Janssen, and Novartis; and has been a consultant for AbbVie, Amgen, Janssen, Lilly, Novartis, Pfizer, and Sandoz. HK has received grant/research support from AbbVie, Asahi-Kasei, Boehringer Ingelheim, Chugai, Eisai, and Mitsubishi-Tanabe; consulting fees from AbbVie, Janssen, Lilly, Novartis, Sanofi, and UCB; and received speakers’ bureau fees from AbbVie, Asahi-Kasei, BMS, Chugai, Eisai, Janssen, Lilly, Mitsubishi-Tanabe, Novartis, and Pfizer. XZ has none declared. YS, XB, PW and I-HS are employees of AbbVie and may own stock or options. ALP is a former employee of AbbVie and may own stock or options.
Figures


References
-
- Boel A, Molto A, van der Heijde D, et al. . Do patients with axial spondyloarthritis with radiographic sacroiliitis fulfil both the modified New York criteria and the ASAS axial spondyloarthritis criteria? Results from eight cohorts. Ann Rheum Dis 2019;78:1545–9. 10.1136/annrheumdis-2019-215707 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

