Keratinocyte-derived S100A9 modulates neutrophil infiltration and affects psoriasis-like skin and joint disease
- PMID: 35788494
- PMCID: PMC9484400
- DOI: 10.1136/annrheumdis-2022-222229
Keratinocyte-derived S100A9 modulates neutrophil infiltration and affects psoriasis-like skin and joint disease
Abstract
Objectives: S100A9, an alarmin that can form calprotectin (CP) heterodimers with S100A8, is mainly produced by keratinocytes and innate immune cells. The contribution of keratinocyte-derived S100A9 to psoriasis (Ps) and psoriatic arthritis (PsA) was evaluated using mouse models, and the potential usefulness of S100A9 as a Ps/PsA biomarker was assessed in patient samples.
Methods: Conditional S100A9 mice were crossed with DKO* mice, an established psoriasis-like mouse model based on inducible epidermal deletion of c-Jun and JunB to achieve additional epidermal deletion of S100A9 (TKO* mice). Psoriatic skin and joint disease were evaluated in DKO* and TKO* by histology, microCT, RNA and proteomic analyses. Furthermore, S100A9 expression was analysed in skin, serum and synovial fluid samples of patients with Ps and PsA.
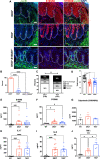
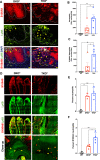
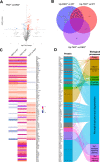
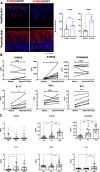
Results: Compared with DKO* littermates, TKO* mice displayed enhanced skin disease severity, PsA incidence and neutrophil infiltration. Altered epidermal expression of selective pro-inflammatory genes and pathways, increased epidermal phosphorylation of STAT3 and higher circulating TNFα were observed in TKO* mice. In humans, synovial S100A9 levels were higher than the respective serum levels. Importantly, patients with PsA had significantly higher serum concentrations of S100A9, CP, VEGF, IL-6 and TNFα compared with patients with only Ps, but only S100A9 and CP could efficiently discriminate healthy individuals, patients with Ps and patients with PsA.
Conclusions: Keratinocyte-derived S100A9 plays a regulatory role in psoriatic skin and joint disease. In humans, S100A9/CP is a promising marker that could help in identifying patients with Ps at risk of developing PsA.
Keywords: cytokines; inflammation; psoriatic arthritis.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


Comment in
-
A protective role for epidermal S100A9 in PsA.Nat Rev Rheumatol. 2022 Sep;18(9):495. doi: 10.1038/s41584-022-00821-4. Nat Rev Rheumatol. 2022. PMID: 35918611 No abstract available.
References
-
- Slobodin G, Rosner I, Rozenbaum M, et al. . Psoriatic arthropathy: where now? Isr Med Assoc J 2009;11:430–4. - PubMed
-
- Gottlieb A, Korman NJ, Gordon KB, et al. . Guidelines of care for the management of psoriasis and psoriatic arthritis: section 2. psoriatic arthritis: overview and guidelines of care for treatment with an emphasis on the biologics. J Am Acad Dermatol 2008;58:851–64. 10.1016/j.jaad.2008.02.040 - DOI - PubMed
-
- Mease PJ. Psoriatic arthritis - update on pathophysiology, assessment, and management. Bull NYU Hosp Jt Dis 2010;68:191–8. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

