Pythium insidiosum Keratitis: Past, Present, and Future
- PMID: 35788551
- PMCID: PMC9255487
- DOI: 10.1007/s40123-022-00542-7
Pythium insidiosum Keratitis: Past, Present, and Future
Abstract
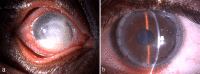
Pythium insidiosum (PI) is an oomycete, a protist belonging to the clade Stramenopila. PI causes vision-threatening keratitis closely mimicking fungal keratitis (FK), hence it is also labeled as "parafungus". PI keratitis was initially confined to Thailand, USA, China, and Australia, but with growing clinical awareness and improvement in diagnostic modalities, the last decade saw a massive upsurge in numbers with the majority of reports coming from India. In the early 1990s, pythiosis was classified as vascular, cutaneous, gastrointestinal, systemic, and ocular. Clinically, morphologically, and microbiologically, PI keratitis closely resembles severe FK and requires a high index of clinical suspicion for diagnosis. The clinical features such as reticular dot infiltrate, tentacular projections, peripheral thinning with guttering, and rapid limbal spread distinguish it from other microorganisms. Routine smearing with Gram and KOH stain reveals perpendicular septate/aseptate hyphae, which closely mimic fungi and make the diagnosis cumbersome. The definitive diagnosis is the presence of dull grey/brown refractile colonies along with zoospore formation upon culture by leaf induction method. However, culture is time-consuming, and currently polymerase chain reaction (PCR) method is the gold standard. The value of other diagnostic modalities such as confocal microscopy and immunohistopathological assays is limited due to cost, non-availability, and limited diagnostic accuracy. PI keratitis is a relatively rare disease without established treatment protocols. Because of its resemblance to fungus, it was earlier treated with antifungals but with an improved understanding of its cell wall structure and absence of ergosterol, this is no longer recommended. Currently, antibacterials have shown promising results. Therapeutic keratoplasty with good margin (1 mm) is mandated for non-resolving cases and corneal perforation. In this review, we have deliberated on the evolution of PI keratitis, covered all the recently available literature, described our current understanding of the diagnosis and treatment, and the potential future diagnostic and management options for PI keratitis.
Keywords: Azithromycin; Keratitis; Leaf incarnation method; Linezolid; Parafungus; Pythium insidiosum; Therapeutic keratoplasty; Zoospore.
© 2022. The Author(s).
Figures


References
-
- Waterhouse GM. Key to Pythium Pringsheim. Mycol Pap. 1967;109:1–15.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

