Mortality and adverse events of hemoadsorption with CytoSorb® in critically ill patients: A systematic review and meta-analysis of randomized controlled trials
- PMID: 35788557
- PMCID: PMC9541789
- DOI: 10.1111/aas.14115
Mortality and adverse events of hemoadsorption with CytoSorb® in critically ill patients: A systematic review and meta-analysis of randomized controlled trials
Abstract
Background: The effects and safety of extracorporeal hemoadsorption with CytoSorb® in critically ill patients with inflammatory conditions are controversial.
Methods: We performed a systematic review with meta-analysis and trial sequential analysis (TSA) of randomized-controlled trials to assess the mortality and safety of CytoSorb® therapy in critically ill patients with inflammatory conditions. Electronic databases were searched up to April 2022. The primary outcome was mortality at longest follow-up and secondary outcomes included various adverse event (AE) outcomes. Conflict of interest and funding of each trial were assessed. We calculated relative risk (RR) and 95% confidence interval (CI).
Results: Fourteen published (n = 764) and 4 unpublished (n = 111) trials were included. Eight trials were performed in medical ICU patients and 10 in complex cardiac surgery. Ten trials had significant industrial funding or an author conflict of interest. Hemoadsorption with CytoSorb® was associated with higher mortality at latest follow-up (16 trials, n = 807, 120 of 402 [29.85%] patients in the CytoSorb® group vs. 98 of 405 [24.20%] patients in the control group, RR = 1.24 [95% CI, 1.04-1.49], p = .02, [TSA-adjusted CI, 0.92-1.68]) and at 30-days or in-hospital (11 trials, n = 727; RR = 1.41 [95% CI, 1.06-1.88], p = .02, [TSA-adjusted CI, 0.44-4.62]). Only one trial reported the definition of adverse event, while detailed results were reported in 3 trials; the risk of adverse events was not higher with CytoSorb®. Certainty of evidence ranged from low to very low.
Conclusion: Low certainty of evidence showed that the use of CytoSorb® might increase mortality in critically ill patients with inflammatory conditions. Adverse events were frequent but underreported and not systematically evaluated. Industrial funding and conflict of interest were common. Considerable uncertainty about the findings does not allow firm conclusions and suggests a need for high-quality randomized trials to clarify mortality and adverse events related to CytoSorb®.
Editorial comment: Hemoadsorption with CytoSorb® have been used in critically ill patients despite lack of high quality data from RCTs suggesting any patient-important benefits. The findings from this systematic review and meta-analysis suggests an increased risk of adverse events including mortality. With no apparent benefits and at the same time risk of harm, use of hemoadsorption with CytoSorb® in daily clinical practice cannot be recommended at this time.
Keywords: CytoSorb; Hemoadsorption; adverse events; hemoperfusion; mortality; safety.
© 2022 The Authors. Acta Anaesthesiologica Scandinavica published by John Wiley & Sons Ltd on behalf of Acta Anaesthesiologica Scandinavica Foundation.
Figures
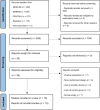
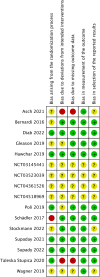



Similar articles
-
Interventions for necrotizing soft tissue infections in adults.Cochrane Database Syst Rev. 2018 May 31;5(5):CD011680. doi: 10.1002/14651858.CD011680.pub2. Cochrane Database Syst Rev. 2018. PMID: 29851032 Free PMC article.
-
Electronic cigarettes for smoking cessation.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD010216. doi: 10.1002/14651858.CD010216.pub7. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 Jan 8;1:CD010216. doi: 10.1002/14651858.CD010216.pub8. PMID: 36384212 Free PMC article. Updated.
-
Topical clonidine for neuropathic pain in adults.Cochrane Database Syst Rev. 2022 May 19;5(5):CD010967. doi: 10.1002/14651858.CD010967.pub3. Cochrane Database Syst Rev. 2022. PMID: 35587172 Free PMC article.
-
Electronic cigarettes for smoking cessation.Cochrane Database Syst Rev. 2021 Sep 14;9(9):CD010216. doi: 10.1002/14651858.CD010216.pub6. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 Nov 17;11:CD010216. doi: 10.1002/14651858.CD010216.pub7. PMID: 34519354 Free PMC article. Updated.
-
Automated monitoring compared to standard care for the early detection of sepsis in critically ill patients.Cochrane Database Syst Rev. 2018 Jun 25;6(6):CD012404. doi: 10.1002/14651858.CD012404.pub2. Cochrane Database Syst Rev. 2018. PMID: 29938790 Free PMC article.
Cited by
-
Vasoplegic Syndrome after Cardiopulmonary Bypass in Cardiovascular Surgery: Pathophysiology and Management in Critical Care.J Clin Med. 2022 Oct 29;11(21):6407. doi: 10.3390/jcm11216407. J Clin Med. 2022. PMID: 36362635 Free PMC article. Review.
-
Hemoadsorption in Multiorgan Failure Due to Viscerocutaneous Loxoscelism.Medicina (Kaunas). 2025 Jan 16;61(1):143. doi: 10.3390/medicina61010143. Medicina (Kaunas). 2025. PMID: 39859124 Free PMC article.
-
Potential Clinical Use of CytoSorb® for Ticagrelor and Rivaroxaban Elimination Prior to Emergency Orthopedic Surgery in Trauma Patients.Life (Basel). 2025 Jul 3;15(7):1065. doi: 10.3390/life15071065. Life (Basel). 2025. PMID: 40724567 Free PMC article.
-
Extracorporeal organ support for critically ill patients: Overcoming the past, achieving the maximum at present, and redefining the future.World J Crit Care Med. 2024 Jun 9;13(2):92458. doi: 10.5492/wjccm.v13.i2.92458. eCollection 2024 Jun 9. World J Crit Care Med. 2024. PMID: 38855267 Free PMC article.
-
Extracorporeal Cytokine Adsorption in Sepsis: Current Evidence and Future Perspectives.Biomedicines. 2025 Jul 9;13(7):1684. doi: 10.3390/biomedicines13071684. Biomedicines. 2025. PMID: 40722757 Free PMC article. Review.
References
-
- Bonavia A, Groff A, Karamchandani K, Singbartl K. Clinical utility of extracorporeal cytokine hemoadsorption therapy: a literature review. Blood Purif. 2018;46:337‐349. - PubMed
-
- Schetz M, Bein T. When more could be industry‐driven: the case of the extracorporeal treatment of sepsis. Intensive Care Med. 2019;45:1622‐1625. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

