High-Dose Dexamethasone and Oxygen Support Strategies in Intensive Care Unit Patients With Severe COVID-19 Acute Hypoxemic Respiratory Failure: The COVIDICUS Randomized Clinical Trial
- PMID: 35788622
- PMCID: PMC9449796
- DOI: 10.1001/jamainternmed.2022.2168
High-Dose Dexamethasone and Oxygen Support Strategies in Intensive Care Unit Patients With Severe COVID-19 Acute Hypoxemic Respiratory Failure: The COVIDICUS Randomized Clinical Trial
Abstract
Importance: The benefit of high-dose dexamethasone and oxygenation strategies vs standard of care for patients with severe acute hypoxemic respiratory failure (AHRF) caused by COVID-19 pneumonia is debated.
Objectives: To assess the benefit of high-dose dexamethasone compared with standard of care dexamethasone, and to assess the benefit of high-flow nasal oxygen (HFNo2) or continuous positive airway pressure (CPAP) compared with oxygen support standard of care (o2SC).
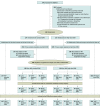
Design, setting, and participants: This multicenter, placebo-controlled randomized clinical trial was conducted in 19 intensive care units (ICUs) in France from April 2020 to January 2021. Eligible patients were consecutive ICU-admitted adults with COVID-19 AHRF. Randomization used a 2 × 3 factorial design for dexamethasone and oxygenation strategies; patients not eligible for at least 1 oxygenation strategy and/or already receiving invasive mechanical ventilation (IMV) were only randomized for dexamethasone. All patients were followed-up for 60 days. Data were analyzed from May 26 to July 31, 2021.
Interventions: Patients received standard dexamethasone (dexamethasone-phosphate 6 mg/d for 10 days [or placebo prior to RECOVERY trial results communication]) or high-dose dexamethasone (dexamethasone-phosphate 20 mg/d on days 1-5 then 10 mg/d on days 6-10). Those not requiring IMV were additionally randomized to o2SC, CPAP, or HFNo2.
Main outcomes and measures: The main outcomes were time to all-cause mortality, assessed at day 60, for the dexamethasone interventions, and time to IMV requirement, assessed at day 28, for the oxygenation interventions. Differences between intervention groups were calculated using proportional Cox models and expressed as hazard ratios (HRs).
Results: Among 841 screened patients, 546 patients (median [IQR] age, 67.4 [59.3-73.1] years; 414 [75.8%] men) were randomized between standard dexamethasone (276 patients, including 37 patients who received placebo) or high-dose dexamethasone (270 patients). Of these, 333 patients were randomized among o2SC (109 patients, including 56 receiving standard dexamethasone), CPAP (109 patients, including 57 receiving standard dexamethasone), and HFNo2 (115 patients, including 56 receiving standard dexamethasone). There was no difference in 60-day mortality between standard and high-dose dexamethasone groups (HR, 0.96 [95% CI, 0.69-1.33]; P = .79). There was no significant difference for the cumulative incidence of IMV criteria at day 28 among o2 support groups (o2SC vs CPAP: HR, 1.08 [95% CI, 0.71-1.63]; o2SC vs HFNo2: HR, 1.04 [95% CI, 0.69-1.55]) or 60-day mortality (o2SC vs CPAP: HR, 0.97 [95% CI, 0.58-1.61; o2SC vs HFNo2: HR, 0.89 [95% CI, 0.53-1.47]). Interactions between interventions were not significant.
Conclusions and relevance: In this randomized clinical trial among ICU patients with COVID-19-related AHRF, high-dose dexamethasone did not significantly improve 60-day survival. The oxygenation strategies in patients who were not initially receiving IMV did not significantly modify 28-day risk of IMV requirement.
Trial registration: ClinicalTrials.gov Identifier: NCT04344730; EudraCT: 2020-001457-43.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

