Radionuclide imaging and therapy directed towards the tumor microenvironment: a multi-cancer approach for personalized medicine
- PMID: 35788730
- PMCID: PMC9606105
- DOI: 10.1007/s00259-022-05870-1
Radionuclide imaging and therapy directed towards the tumor microenvironment: a multi-cancer approach for personalized medicine
Abstract
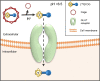
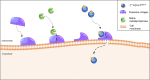
Targeted radionuclide theranostics is becoming more and more prominent in clinical oncology. Currently, most nuclear medicine compounds researched for cancer theranostics are directed towards targets expressed in only a small subset of cancer types, limiting clinical applicability. The identification of cancer-specific targets that are (more) universally expressed will allow more cancer patients to benefit from these personalized nuclear medicine-based interventions. A tumor is not merely a collection of cancer cells, it also comprises supporting stromal cells embedded in an altered extracellular matrix (ECM), together forming the tumor microenvironment (TME). Since the TME is less genetically unstable than cancer cells, and TME phenotypes can be shared between cancer types, it offers targets that are more universally expressed. The TME is characterized by the presence of altered processes such as hypoxia, acidity, and increased metabolism. Next to the ECM, the TME consists of cancer-associated fibroblasts (CAFs), macrophages, endothelial cells forming the neo-vasculature, immune cells, and cancer-associated adipocytes (CAAs). Radioligands directed at the altered processes, the ECM, and the cellular components of the TME have been developed and evaluated in preclinical and clinical studies for targeted radionuclide imaging and/or therapy. In this review, we provide an overview of the TME targets and their corresponding radioligands. In addition, we discuss what developments are needed to further explore the TME as a target for radionuclide theranostics, with the hopes of stimulating the development of novel TME radioligands with multi-cancer, or in some cases even pan-cancer, application.
Keywords: Cancer stroma; Pan-cancer therapy; Radionuclide imaging and therapy; Theranostics; Tumor microenvironment.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Tumor Microenvironment and Nitric Oxide: Concepts and Mechanisms.Adv Exp Med Biol. 2020;1277:143-158. doi: 10.1007/978-3-030-50224-9_10. Adv Exp Med Biol. 2020. PMID: 33119871
-
Architecture of Cancer-Associated Fibroblasts in Tumor Microenvironment: Mapping Their Origins, Heterogeneity, and Role in Cancer Therapy Resistance.OMICS. 2020 Jun;24(6):314-339. doi: 10.1089/omi.2020.0023. OMICS. 2020. PMID: 32496970
-
The tumor microenvironment as driver of stemness and therapeutic resistance in breast cancer: New challenges and therapeutic opportunities.Cell Oncol (Dordr). 2021 Dec;44(6):1209-1229. doi: 10.1007/s13402-021-00634-9. Epub 2021 Sep 16. Cell Oncol (Dordr). 2021. PMID: 34528143 Review.
-
Targeting the microenvironment in solid tumors.Cancer Treat Rev. 2018 Apr;65:22-32. doi: 10.1016/j.ctrv.2018.02.004. Epub 2018 Feb 22. Cancer Treat Rev. 2018. PMID: 29502037 Review.
-
The tumor microenvironment: shaping cancer progression and treatment response.J Chemother. 2025 Feb;37(1):15-44. doi: 10.1080/1120009X.2023.2300224. Epub 2024 Jan 5. J Chemother. 2025. PMID: 38179655 Review.
Cited by
-
In vitro and in vivo analyses of eFAP: a novel FAP-targeting small molecule for radionuclide theranostics and other oncological interventions.EJNMMI Radiopharm Chem. 2024 Jul 29;9(1):55. doi: 10.1186/s41181-024-00283-x. EJNMMI Radiopharm Chem. 2024. PMID: 39073475 Free PMC article.
-
Three-Dimensional Microtumor Formation of Infantile Hemangioma-Derived Endothelial Cells for Mechanistic Exploration and Drug Screening.Pharmaceuticals (Basel). 2022 Nov 12;15(11):1393. doi: 10.3390/ph15111393. Pharmaceuticals (Basel). 2022. PMID: 36422523 Free PMC article.
-
Current status and future prospects of molecular imaging in targeting the tumor immune microenvironment.Front Immunol. 2025 Jan 22;16:1518555. doi: 10.3389/fimmu.2025.1518555. eCollection 2025. Front Immunol. 2025. PMID: 39911388 Free PMC article. Review.
-
Development and Application of Radioactive Ligands Targeting Fibroblasts with Albumin-Binding Sites.Mol Cancer Ther. 2025 Aug 1;24(8):1186-1196. doi: 10.1158/1535-7163.MCT-24-1108. Mol Cancer Ther. 2025. PMID: 40177850 Free PMC article.
-
Exploring the landscape of current in vitro and in vivo models and their relevance for targeted radionuclide theranostics.Eur J Nucl Med Mol Imaging. 2025 Jul;52(9):3291-3311. doi: 10.1007/s00259-025-07123-3. Epub 2025 Feb 28. Eur J Nucl Med Mol Imaging. 2025. PMID: 40016527 Free PMC article. Review.
References
-
- Abadjian MCZ, Edwards WB, Anderson CJ. Imaging the tumor microenvironment. Adv Exp Med Biol; 2017. p. 229–57. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

