Selfish centromeres and the wastefulness of human reproduction
- PMID: 35788750
- PMCID: PMC9255743
- DOI: 10.1371/journal.pbio.3001671
Selfish centromeres and the wastefulness of human reproduction
Abstract
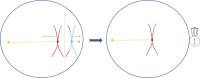
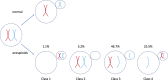
Many human embryos die in utero owing to an excess or deficit of chromosomes, a phenomenon known as aneuploidy; this is largely a consequence of nondisjunction during maternal meiosis I. Asymmetries of this division render it vulnerable to selfish centromeres that promote their own transmission, these being thought to somehow underpin aneuploidy. In this essay, I suggest that these vulnerabilities provide only half the solution to the enigma. In mammals, as in utero and postnatal provisioning is continuous, the costs of early death are mitigated. With such reproductive compensation, selection can favour a centromere because it induces lethal aneuploidy: if, when taken towards the polar body, it instead kills the embryo via aneuploidy, it gains. The model is consistent with the observation that reduced dosage of a murine drive suppressor induces aneuploidy and with the fact that high aneuploidy rates in vertebrates are seen exclusively in mammals. I propose further tests of this idea. The wastefulness of human reproduction may be a price we pay for nurturing our offspring.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Molecular Strategies of Meiotic Cheating by Selfish Centromeres.Cell. 2019 Aug 22;178(5):1132-1144.e10. doi: 10.1016/j.cell.2019.07.001. Epub 2019 Aug 8. Cell. 2019. PMID: 31402175 Free PMC article.
-
Embryos whose polar bodies contain isolated reciprocal chromosome aneuploidy are almost always euploid.Hum Reprod. 2013 Feb;28(2):502-8. doi: 10.1093/humrep/des393. Epub 2012 Nov 20. Hum Reprod. 2013. PMID: 23169867 Free PMC article.
-
Sequence of centromere separation another mechanism for the origin of nondisjunction.Hum Genet. 1984;66(2-3):239-43. doi: 10.1007/BF00286609. Hum Genet. 1984. PMID: 6232200
-
Adaptations for centromere function in meiosis.Essays Biochem. 2020 Sep 4;64(2):193-203. doi: 10.1042/EBC20190076. Essays Biochem. 2020. PMID: 32406496 Free PMC article. Review.
-
Meiotic drive of noncentromeric loci in mammalian meiosis II eggs.Curr Opin Genet Dev. 2023 Aug;81:102082. doi: 10.1016/j.gde.2023.102082. Epub 2023 Jul 3. Curr Opin Genet Dev. 2023. PMID: 37406428 Free PMC article. Review.
Cited by
-
Early-acting inbreeding depression can evolve as an inbreeding avoidance mechanism.Proc Biol Sci. 2024 Mar 13;291(2018):20232467. doi: 10.1098/rspb.2023.2467. Epub 2024 Mar 6. Proc Biol Sci. 2024. PMID: 38444336 Free PMC article.
-
Mendel's legacy in modern genetics.PLoS Biol. 2022 Jul 28;20(7):e3001760. doi: 10.1371/journal.pbio.3001760. eCollection 2022 Jul. PLoS Biol. 2022. PMID: 35901028 Free PMC article.
-
Juvenile mortality and sibling replacement: a kin selection approach.Evol Lett. 2024 May 6;8(5):647-657. doi: 10.1093/evlett/qrae018. eCollection 2024 Oct. Evol Lett. 2024. PMID: 39957726 Free PMC article.
-
Envisioning a new era: Complete genetic information from routine, telomere-to-telomere genomes.Am J Hum Genet. 2023 Nov 2;110(11):1832-1840. doi: 10.1016/j.ajhg.2023.09.011. Am J Hum Genet. 2023. PMID: 37922882 Free PMC article. Review.
-
Analysis of copy number variations and possible candidate genes in spontaneous abortion by copy number variation sequencing.Front Endocrinol (Lausanne). 2023 Oct 17;14:1218793. doi: 10.3389/fendo.2023.1218793. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37916154 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

