A new adenine nucleotide transporter located in the ER is essential for maintaining the growth of Toxoplasma gondii
- PMID: 35788770
- PMCID: PMC9286291
- DOI: 10.1371/journal.ppat.1010665
A new adenine nucleotide transporter located in the ER is essential for maintaining the growth of Toxoplasma gondii
Erratum in
-
Correction: A new adenine nucleotide transporter located in the ER is essential for maintaining the growth of Toxoplasma gondii.PLoS Pathog. 2022 Nov 29;18(11):e1010998. doi: 10.1371/journal.ppat.1010998. eCollection 2022 Nov. PLoS Pathog. 2022. PMID: 36445866 Free PMC article.
Abstract
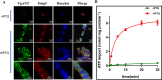
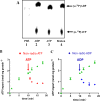
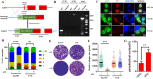
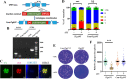
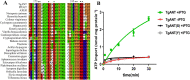
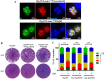
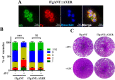
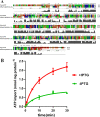
The lumen of the endoplasmic reticulum (ER) is the subcellular site where secretory protein folding, glycosylation and sulfation of membrane-bound proteins, proteoglycans, and lipids occur. The protein folding and degradation in the lumen of the ER require high levels of energy in the form of ATP. Biochemical and genetic approaches show that ATP must first be translocated across ER membrane by particular transporters before serving as substrates and energy sources in the lumenal reactions. Here we describe an ATP/ADP transporter residing in the ER membranes of T.gondii. Immunofluorescence (IFA) assay in transgenic TgANT1-HA tag revealed that TgANT1 is a protein specifically expressed in the ER. In vitro assays, functional integration of TgANT in the cytoplasmic membrane of intact E. coli cells reveals high specificity for an ATP/ADP antiport. The depletion of TgANT leads to fatal growth defects in T.gondii, including a significant slowdown in replication, no visible plaque formation, and reduced ability to invade. We also found that the amino acid mutations in two domains of TgANT lead to the complete loss of its function. Since these two domains are conserved in multiple species, they may share the same transport mechanism. Our results indicate that TgANT is the only ATP/ADP transporter in the ER of T. gondii, and the lack of ATP in the ER is the cause of the death of T. gondii.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Identification of a novel adenine nucleotide transporter in the endoplasmic reticulum of Arabidopsis.Plant Cell. 2008 Feb;20(2):438-51. doi: 10.1105/tpc.107.057554. Epub 2008 Feb 22. Plant Cell. 2008. PMID: 18296626 Free PMC article.
-
Depletion of Toxoplasma adenine nucleotide translocator leads to defects in mitochondrial morphology.Parasit Vectors. 2022 May 31;15(1):185. doi: 10.1186/s13071-022-05295-7. Parasit Vectors. 2022. PMID: 35642006 Free PMC article.
-
AXER is an ATP/ADP exchanger in the membrane of the endoplasmic reticulum.Nat Commun. 2018 Aug 28;9(1):3489. doi: 10.1038/s41467-018-06003-9. Nat Commun. 2018. PMID: 30154480 Free PMC article.
-
Transporters of nucleotide sugars, ATP, and nucleotide sulfate in the endoplasmic reticulum and Golgi apparatus.Annu Rev Biochem. 1998;67:49-69. doi: 10.1146/annurev.biochem.67.1.49. Annu Rev Biochem. 1998. PMID: 9759482 Review.
-
Nucleotide sugars, nucleotide sulfate, and ATP transporters of the endoplasmic reticulum and Golgi apparatus.Ann N Y Acad Sci. 1998 Apr 15;842:91-9. doi: 10.1111/j.1749-6632.1998.tb09636.x. Ann N Y Acad Sci. 1998. PMID: 9599298 Review.
Cited by
-
TgMORN2, a MORN Family Protein Involved in the Regulation of Endoplasmic Reticulum Stress in Toxoplasma gondii.Int J Mol Sci. 2023 Jun 16;24(12):10228. doi: 10.3390/ijms241210228. Int J Mol Sci. 2023. PMID: 37373373 Free PMC article.
-
Functional dissection of prenyltransferases reveals roles in endocytosis and secretory vacuolar sorting in type 2-ME49 strain of Toxoplasma gondii.Virulence. 2024 Dec;15(1):2432681. doi: 10.1080/21505594.2024.2432681. Epub 2024 Nov 26. Virulence. 2024. PMID: 39569525 Free PMC article.
-
Molecular and cellular characterization of four putative nucleotide transporters from the shrimp microsporidian Enterocytozoon hepatopenaei (EHP).Sci Rep. 2023 Nov 16;13(1):20008. doi: 10.1038/s41598-023-47114-8. Sci Rep. 2023. PMID: 37974017 Free PMC article.
-
Mitochondrial ADP/ATP Carrier 1 Is Important for the Growth of Toxoplasma Tachyzoites.Microbiol Spectr. 2023 Jun 15;11(3):e0004023. doi: 10.1128/spectrum.00040-23. Epub 2023 May 8. Microbiol Spectr. 2023. PMID: 37154708 Free PMC article.
-
Correction: A new adenine nucleotide transporter located in the ER is essential for maintaining the growth of Toxoplasma gondii.PLoS Pathog. 2022 Nov 29;18(11):e1010998. doi: 10.1371/journal.ppat.1010998. eCollection 2022 Nov. PLoS Pathog. 2022. PMID: 36445866 Free PMC article.
References
-
- Carmeille R, Lomoriello PS, Devarakonda PM, Kellermeier JA, Heaslip AT. Actin and an unconventional myosin motor, TgMyoF, control the organization and dynamics of the endomembrane network in Toxoplasma gondii. Plos Pathogens. 2021;17(2). ARTN e1008787 doi: 10.1371/journal.ppat.1008787 WOS:000616583700001. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

