Mechanisms underlying synergism between circularized tumor necrosis factor-related apoptosis inducing ligand and bortezomib in bortezomib-sensitive or -resistant myeloma cells
- PMID: 35789025
- PMCID: PMC10084357
- DOI: 10.1002/hon.3045
Mechanisms underlying synergism between circularized tumor necrosis factor-related apoptosis inducing ligand and bortezomib in bortezomib-sensitive or -resistant myeloma cells
Abstract
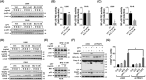
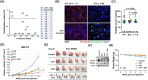
Mechanisms underlying interactions between a novel, clinically relevant circularized tumor necrosis factor-related apoptosis inducing ligand (TRAIL) agonist, circularly permuted TRAIL (CPT) have been examined in multiple myeloma (MM) cells sensitive or resistant to bortezomib (BTZ). Various MM cell lines for example, U266, including those resistant to bortezomib-resistant U266 cells were exposed to low nanomolar concentrations of bortezomib ± CPT and apoptosis monitored. Circularly permuted TRAIL and bortezomib synergistically induced apoptosis in both BTZ-naïve and -resistant cells. The regimen up-regulated DR4 receptor internalization in MM cells, known to modulate both NF-κB and extrinsic apoptotic pathways. CPT/BTZ disrupted the non-canonical NF-κB pathway, reflected by tumor necrosis factor (TNF) receptor associated factors 3 (TRAF3) up-regulation, NF-κB inducing kinase down-regulation, diminished p52 and p50 processing, and B-cell lymphoma-extra large (BCL-XL) down-regulation, but failed to inactivate the canonical NF-κB pathway, reflected by unchanged or increased expression of phospho-p65. The regimen also sharply increased extrinsic apoptotic pathway activation. Cells exhibiting TRAF3 knock-down, dominant-negative Fas-associated protein with death domain, knock-down of caspase-8, BCL-2/BCL-XL, or exposure to a caspase-9 inhibitor displayed markedly reduced CPT/BTZ sensitivity. Concordant results were observed in bortezomib-resistant cells. The regimen was also active in the presence of stromal cells and was relatively sparing toward normal CD34+ hematopoietic cells. Finally, ex vivo results revealed synergism in primary MM primary cells, including those BTZ, and the CPT/BTZ regimen significantly decreased tumor growth in a patient-derived MM xenograft model. These results indicate that the CPT/BTZ regimen acts via the non-canonical NF-κB as well as intrinsic/extrinsic apoptotic pathways to induce cell death in MM cells, and may represent an effective strategy in the setting of bortezomib resistance.
Keywords: CPT; TRAIL; bortezomib; intrinsic/extrinsic apoptotic; multiple myeloma; non-canonical NF-κB pathway.
© 2022 The Authors. Hematological Oncology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
The IAP antagonist birinapant potentiates bortezomib anti-myeloma activity in vitro and in vivo.J Hematol Oncol. 2019 Mar 7;12(1):25. doi: 10.1186/s13045-019-0713-x. J Hematol Oncol. 2019. PMID: 30845975 Free PMC article.
-
Mechanisms underlying reversed TRAIL sensitivity in acquired bortezomib-resistant non-small cell lung cancer cells.Cancer Drug Resist. 2024 Apr 9;7:12. doi: 10.20517/cdr.2024.14. eCollection 2024. Cancer Drug Resist. 2024. PMID: 38835345 Free PMC article.
-
TM-233, a novel analog of 1'-acetoxychavicol acetate, induces cell death in myeloma cells by inhibiting both JAK/STAT and proteasome activities.Cancer Sci. 2015 Apr;106(4):438-46. doi: 10.1111/cas.12616. Epub 2015 Mar 10. Cancer Sci. 2015. PMID: 25613668 Free PMC article.
-
IAP and HDAC inhibitors interact synergistically in myeloma cells through noncanonical NF-κB- and caspase-8-dependent mechanisms.Blood Adv. 2021 Oct 12;5(19):3776-3788. doi: 10.1182/bloodadvances.2020003597. Blood Adv. 2021. PMID: 34464977 Free PMC article.
-
Epigenetic Alterations as Vital Aspects of Bortezomib Molecular Action.Cancers (Basel). 2023 Dec 23;16(1):84. doi: 10.3390/cancers16010084. Cancers (Basel). 2023. PMID: 38201512 Free PMC article. Review.
Cited by
-
Aponermin: First Approval.Drugs. 2024 Apr;84(4):459-466. doi: 10.1007/s40265-024-02004-9. Drugs. 2024. PMID: 38441805 Review.
-
GZ17-6.02 interacts with proteasome inhibitors to kill multiple myeloma cells.Oncotarget. 2024 Mar 5;15:159-174. doi: 10.18632/oncotarget.28558. Oncotarget. 2024. PMID: 38441437 Free PMC article.
-
Non-canonical role for the ataxia-telangiectasia-Rad3 pathway in STAT3 activation in human multiple myeloma cells.Cell Oncol (Dordr). 2023 Oct;46(5):1369-1380. doi: 10.1007/s13402-023-00817-6. Epub 2023 May 1. Cell Oncol (Dordr). 2023. PMID: 37126127 Free PMC article.
References
-
- Albagoush SA, Azevedo AM. Multiple Myeloma. StatPearls. Treasure Island (FL); 2020.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

