HDACs regulate the differentiation of endothelial cells from human iPSCs
- PMID: 35789099
- PMCID: PMC9391285
- DOI: 10.1002/cbf.3729
HDACs regulate the differentiation of endothelial cells from human iPSCs
Abstract
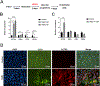
Human induced pluripotent stem cells (hiPSCs) possess the potential to differentiate toward vascular cells including endothelial cells (ECs), pericytes, and smooth muscle cells. Epigenetic mechanisms including DNA methylation and histone modification play a crucial role in regulating lineage differentiation and specification. Herein, we utilized a three-stage protocol to induce differentiation of mesoderm, vascular progenitors, and ECs from hiPSCs and investigated the regulatory effects of histone acetylation on the differentiation processes. We found that the expression of several histone deacetylases (HDACs), including HDAC1, HDAC5, and HDAC7, were greatly upregulated at the second stage and downregulated at the third stage. Interestingly, although HDAC1 remained in the nucleus during the EC differentiation, HDAC5 and HDAC7 displayed cytosol/nuclear translocation during the differentiation process. Inhibition of HDACs with sodium butyrate (NaBt) or BML210 could hinder the differentiation of vascular progenitors at the second stage and facilitate EC induction at the third stage. Further investigation revealed that HDAC may modulate the stepwise EC differentiation via regulating the expression of endothelial transcription factors ERG, ETS1, and MEF2C. Opposite to the expression of EC markers, the smooth muscle/pericyte marker ACTA2 was upregulated at the second stage and downregulated at the third stage by NaBt. The stage-specific regulation of ACTA2 by HDAC inhibition was likely through regulating the expression of TGFβ2 and PDGFB. This study suggests that HDACs play different roles at different stages of EC induction by promoting the commitment of vascular progenitors and impeding the later stage differentiation of ECs.
Keywords: HDAC inhibitor; differentiation; endothelial cells; histone deacetylase; induced pluripotent stem cells.
© 2022 John Wiley & Sons Ltd.
Conflict of interest statement
Competing interests
The authors declare there are no competing interests
Figures

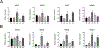
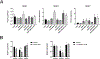

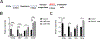
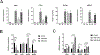
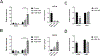
Similar articles
-
Highly Efficient Differentiation of Endothelial Cells from Pluripotent Stem Cells Requires the MAPK and the PI3K Pathways.Stem Cells. 2017 Apr;35(4):909-919. doi: 10.1002/stem.2577. Epub 2017 Mar 1. Stem Cells. 2017. PMID: 28248004
-
Histone deacetylase 1 and 3 regulate the mesodermal lineage commitment of mouse embryonic stem cells.PLoS One. 2014 Nov 20;9(11):e113262. doi: 10.1371/journal.pone.0113262. eCollection 2014. PLoS One. 2014. PMID: 25412078 Free PMC article.
-
Role of histone deacetylases in vascular cell homeostasis and arteriosclerosis.Cardiovasc Res. 2011 Jun 1;90(3):413-20. doi: 10.1093/cvr/cvr003. Epub 2011 Jan 13. Cardiovasc Res. 2011. PMID: 21233251 Review.
-
Functionality of endothelial cells and pericytes from human pluripotent stem cells demonstrated in cultured vascular plexus and zebrafish xenografts.Arterioscler Thromb Vasc Biol. 2014 Jan;34(1):177-86. doi: 10.1161/ATVBAHA.113.302598. Epub 2013 Oct 24. Arterioscler Thromb Vasc Biol. 2014. PMID: 24158517
-
[Epigenetic mechanisms and alcohol use disorders: a potential therapeutic target].Biol Aujourdhui. 2017;211(1):83-91. doi: 10.1051/jbio/2017014. Epub 2017 Jul 6. Biol Aujourdhui. 2017. PMID: 28682229 Review. French.
References
-
- Lin Y, Gil CH, Yoder MC. Differentiation, Evaluation, and Application of Human Induced Pluripotent Stem Cell-Derived Endothelial Cells. Arteriosclerosis, thrombosis, and vascular biology. 2017;37(11):2014–2025. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

