Impact of Hepatitis C Virus Cure on Depressive Symptoms in the Human Immunodeficiency Virus-Hepatitis C Virus Coinfected Population in Canada
- PMID: 35789253
- PMCID: PMC9907551
- DOI: 10.1093/cid/ciac540
Impact of Hepatitis C Virus Cure on Depressive Symptoms in the Human Immunodeficiency Virus-Hepatitis C Virus Coinfected Population in Canada
Abstract
Background: Depression is common in people with human immunodeficiency virus (HIV) and hepatitis C virus (HCV), with biological and psychosocial mechanisms at play. Direct acting antivirals (DAA) result in high rates of sustained virologic response (SVR), with minimal side-effects. We assessed the impact of SVR on presence of depressive symptoms in the HIV-HCV coinfected population in Canada during the second-generation DAA era (2013-2020).
Methods: We used data from the Canadian CoInfection Cohort (CCC), a multicenter prospective cohort of people with a HIV and HCV coinfection, and its associated sub-study on food security. Because depression screening was performed only in the sub-study, we predicted Center for Epidemiologic Studies Depression Scale-10 classes in the CCC using a random forest classifier and corrected for misclassification. We included participants who achieved SVR and fit a segmented modified Poisson model using an interrupted time series design, adjusting for time-varying confounders.
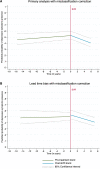
Results: We included 470 participants; 58% had predicted depressive symptoms at baseline. The median follow-up was 2.4 years (interquartile range [IQR]: 1.0-4.5.) pre-SVR and 1.4 years (IQR: 0.6-2.5) post-SVR. The pre-SVR trend suggested depressive symptoms changed little over time, with no immediate level change at SVR. However, post-SVR trends showed a reduction of 5% per year (risk ratio: 0.95 (95% confidence interval [CI]: .94-.96)) in the prevalence of depressive symptoms.
Conclusions: In the DAA era, predicted depressive symptoms declined over time following SVR. These improvements reflect possible changes in biological pathways and/or better general health. If such improvements in depression symptoms are durable, this provides an additional reason for treatment and early cure of HCV.
Keywords: HCV cure; HIV-HCV coinfection; depressive symptoms; direct acting antivirals; sustained virologic response.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. J. C. received grants and consulting fees from ViiV Healthcare, Merck, and Gilead and personal fees from Bristol-Myers Squibb and reports payment or honoraria from Gilead Sciences for a presentation and support from Gilead Sciences for conference travel. J. G. has served as ad hoc member on National HIV advisory boards to ViiV healthcare, Gilead, and Merck. C. C. has received personal fees for being a member of the national advisory boards, consulting fees, payment or honoraria, and/or support for attending meetings and/or travel of Gilead, Merck, Janssen, Abbvie, MK, and Bristol-Myers Squibb. S. W. received grants, consulting fees, lecture fees, nonfinancial support, and fees for the development of educational presentations from Merck, ViiV Healthcare, GlaxoSmithKline, Pfizer, Gilead, AbbVie, Bristol-Myers Squibb, and Janssen. N. P. reports honoraria from Gilead and ViiV Healthcare. M. B. K. reports grants for investigator-initiated studies from ViiV Healthcare, AbbVie, Merck, and Gilead; and consulting fees from ViiV Healthcare, Merck, AbbVie, and Gilead. G. M. reports support for attending meetings and/or travel from CROI new investigator scholarship 2022. M. L. V. reports grant or contracts for Clinical Trials from AbbVie and Merck outside of the submitted work and consulting fees from AbbVie, Gilead, and Merck. V. M. L. reports grants or contracts outside of the submitted work from Gilead Science and Merck and consulting fees and payment or honoraria from Abbvie. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

