Surgical aortic valve replacement with a stented pericardial bioprosthesis: 5-year outcomes
- PMID: 35789382
- PMCID: PMC9346377
- DOI: 10.1093/ejcts/ezac374
Surgical aortic valve replacement with a stented pericardial bioprosthesis: 5-year outcomes
Abstract
Objectives: This analysis evaluated the safety, durability and haemodynamic performance of a stented bovine pericardial valve through 5 years of follow-up in patients with an indication for surgical aortic valve replacement.
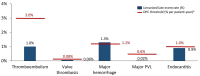
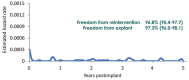
Methods: Kaplan-Meier analysis was used to estimate the incidence of survival and valve-related thromboembolism, major paravalvular leak, endocarditis, structural valve deterioration (SVD) and reintervention. The mean aortic gradient and New York Heart Association (NYHA) functional class were also evaluated.
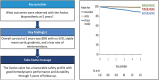
Results: A total of 1118 patients have received the Avalus valve; 564 have completed the 5-year follow-up. The median follow-up was 4.85 years (4810 patient-years total follow-up). At baseline, the mean age was 70.2 ± 9.0 years; 75.1% of patients were male. The Society of Thoracic Surgeons predicted risk of mortality was 2.0 ± 1.4%. Most patients were in NYHA functional class II (46.8%) or III (40.3%). At the 5-year follow-up, the overall Kaplan-Meier survival rate was 88.1% (85.9-90.0%). The Kaplan-Meier event rates were 5.6% (4.3-7.2%) for thromboembolism, 4.4% (3.2-6.0%) for endocarditis, 0.2% (0.0-0.7%) for a major paravalvular leak and 3.2% (2.3-4.6%) for reintervention. There were no cases of SVD. The mean gradient decreased from 42.1 ± 17.1 mmHg at baseline, to 13.1 ± 4.7 mmHg at discharge and remained stable at 12.5 ± 4.6 mmHg at 5 years. More than 95% of patients were in NYHA functional class I/II 5 years after surgery.
Conclusions: The findings of a high survival rate, excellent safety, no SVD and stable haemodynamic performance and functional status through 5 years of follow-up are encouraging. Additional follow-up is needed to assess the long-term durability of this contemporary surgical bioprosthesis.
Keywords: Aortic valve disease; Bovine pericardial aortic bioprosthesis; Cardiac surgery; Surgical aortic valve replacement.
© The Author(s) 2022. Published by Oxford University Press on behalf of the European Association for Cardio-Thoracic Surgery.
Figures



Comment in
-
Commentary: five-year results of a novel surgical bioprosthetic valve look promising, but only time will tell.Eur J Cardiothorac Surg. 2022 Aug 3;62(3):ezac407. doi: 10.1093/ejcts/ezac407. Eur J Cardiothorac Surg. 2022. PMID: 35984311 No abstract available.
References
-
- Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Sondergaard L, Mumtaz M, SURTAVI Investigators et al.Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med 2017;376:1321–31. - PubMed
-
- Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, Evolut Low Risk Trial Investigators et al.Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med 2019;380:1706–15. - PubMed
-
- Sondergaard L, Ihlemann N, Capodanno D, Jorgensen TH, Nissen H, Kjeldsen BJ. et al. Durability of transcatheter and surgical bioprosthetic aortic valves in patients at lower surgical risk. J Am Coll Cardiol 2019;73:546–53. - PubMed
-
- Thyregod HGH, Ihlemann N, Jorgensen TH, Nissen H, Kjeldsen BJ, Petursson P. et al. Five-year clinical and echocardiographic outcomes from the Nordic Aortic Valve Intervention (NOTION) randomized clinical trial in lower surgical risk patients. Circulation 2019;139:2714–23. - PubMed
-
- Carroll JD, Mack MJ, Vemulapalli S, Herrmann HC, Gleason TG, Hanzel G. et al. STS-ACC TVT registry of transcatheter aortic valve replacement. J Am Coll Cardiol 2020;76:2492–516. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

