Comparative analysis of rituximab or obinutuzumab combined with CHOP in first-line treatment of follicular lymphoma
- PMID: 35789429
- PMCID: PMC9255466
- DOI: 10.1007/s00432-022-04155-2
Comparative analysis of rituximab or obinutuzumab combined with CHOP in first-line treatment of follicular lymphoma
Abstract
Purpose: Rituximab (R) or obinutuzumab (G) combined with CHOP chemotherapy are used in previously untreated follicular lymphoma (FL). The aim is to compare in real life setting the efficacy and safety of these therapeutic strategies and assess the economic impact of introducing G.
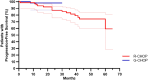
Methods: This retrospective study, performed in 3 centers, included data from all patients who received R-CHOP or G-CHOP for previous untreated FL from June 1st, 2016 to December 31st, 2020. Progression-Free Survival (PFS) were estimated according to the Kaplan-Meier method. A budgetary impact model was performed from the French health care system's perspective.
Results: N = 124 patients were included (58 G-CHOP; 66 R-CHOP). Fifty-one and 57 patients achieved a complete response at the end of induction in the G-CHOP and R-CHOP group, respectively. PFS was not significantly longer in the G-CHOP group (HR 0.28; 95% CI 0.08-0.97; p value = 0.14). Hematological toxicity occurred more frequently with G-CHOP than R-CHOP during induction treatment (n = 58; 100% vs. n = 61; 92%), including higher severe neutropenia (grade ≥ 3) (n = 26; 45% vs. n = 23; 35%). Infusion-related reactions during the first infusion occurred more frequently with G-CHOP (n = 19; 33% vs. n = 16; 24%). The introduction of a completed G treatment (induction and maintenance) results in an additional cumulative cost per patient estimated at more than €30,000.
Conclusion: Similar results were found in the GALLIUM subgroup analysis study, suggesting that at this time there is no absolute benefit to administer G-CHOP instead of R-CHOP in all patients with previously untreated FL and may encourage clinical and economic trials including quality of life data.
Keywords: Drug therapy; Follicular lymphoma; Obinutuzumab; Rituximab.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors declare that they have no conflict of interest.
References
-
- Amitai I, Gafter-Gvili A, Shargian-Alon L, Raanani P, Gurion R (2021) Obinutuzumab-related adverse events: a systematic review and meta-analysis. Hematol Oncol 39(2):215–221. 10.1002/hon.2828 - PubMed
-
- Brice P, Bastion Y, Lepage E, Brousse N, Haïoun C, Moreau P et al (1997) Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: a randomized study from the. Groupe d’Etude des Lymphomes Folliculaires Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol 15(3):1110–1117. 10.1200/JCO.1997.15.3.1110 - PubMed
-
- Casulo C, Byrtek M, Dawson KL, Zhou X, Farber CM, Flowers CR et al (2015) Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the national lymphocare study. J Clin Oncol 33(23):2516–2522. 10.1200/JCO.2014.59.7534 - PMC - PubMed
-
- Casulo C, Leademacher J, Dixon J, Salles G, Hoster E, Herold M et al (2017) Validation of POD24 as a robust early clinical endpoint of poor survival in follicular lymphoma: results from the follicular lymphoma analysis of surrogacy hypothesis (FLASH) investigation using individual data from 5,453 patients on 13 clinical trials. Blood 130(1, Suppl 1):412–412
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials