Effect of seasonal malaria chemoprevention in children between 5 and 9 years old in Kita and Bafoulabe districts, Mali
- PMID: 35789762
- PMCID: PMC9249800
- DOI: 10.1016/j.parepi.2022.e00258
Effect of seasonal malaria chemoprevention in children between 5 and 9 years old in Kita and Bafoulabe districts, Mali
Abstract
Background: Seasonal malaria chemoprevention (SMC) has been widely expanded in Mali since its recommendation by the the World Health Organization in 2012. SMC guidelines currently target children between three months and five years of age. The SMC initiative has been largely successful. Children at least five years of age are not currently covered by current SMC guidelines but bear a considerable portion of the malaria burden. For this reason, this study sought to determine the feasibility and effectiveness for extending SMC to children aged 5-9 years.
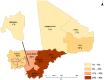
Methods: A non-randomized, pre-post study was performed with an intervention district (Kita) and a comparison district (Bafoulabe). Children aged 3-59 months received SMC in both comparison districts, and children aged 60-120 months received SMC in the intervention district. SMC was delivered as sulfadoxine-pyriméthamine plus amodiaquine (SP-AQ) at monthly intervals from July to October in 2017 and 2018 during the historical transmission seasons. Baseline and endline cross-sectional surveys were conducted in both comparison districts. A total of 200 household surveys were conducted at each of the four monthly SMC cycles to determine adherence and tolerance to SMC in the intervention district.
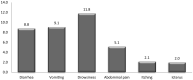
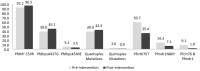
Results: In July 2017, 633 children aged 60-120 months old were enrolled at the Kita and Bafoulabe study sites (n = 310 and n = 323, respectively). Parasitemia prevalence was similar in the intervention and comparison districts prior the SMC campaign (27.7% versus 21.7%, p = 0.07). Mild anemia was observed in 14.2% children in Kita and in 10.5% of children in Bafoulabé. At the Kita site, household surveys showed an SMC coverage rate of 89.1% with a response rate of 93.3% among child caregivers. The most common adverse event reported by parents was drowsiness (11.8%). One year following SMC implementation in the older age group in Kita, the coverage of three doses per round was 81.2%. Between the baseline and endline surveys, there was a reduction in parasitemia prevalence of 40% (OR = 0.60, CI: 0.41-0.89). Malaria molecular resistance was low in the intervention district following the intervention. A significant reduction in the prevalence of parasitemia in children 60 to 120 months was observed in the intervention district, but the prevalance of clinical malaria remained relatively constant.
Conclusion: This study shows that the prospect of extending SMC coverage to children between five and nine years old is encouraging. The reduction in the parasitemia could also warrant consideration for adapting SMC policy to account for extended malaria transmission seasons.
Keywords: AQ, Amodiaquine; CBD, Community based distributors; CHW, Community Health Workers; DHIS2, District Health Information System 2; DHS, Demographic Health Survey; EPI, Expended Program on Immunization; HMIS, Health Management and Information System; IPTi, Intermittent Preventive treatment in Infant; ITN, Insecticide Treat Net; Implementation; LLIN, Long-lasting Insecticide Treated bed Net; Malaria; Mali; Molecular drug resistance; SMC, Seasonal Malaria Chemoprevention; SP, Sulfadoxine-Pyriméthamine; Seasonal malaria chemoprevention; WHO, World Health Organization.
© 2022 Published by Elsevier Ltd on behalf of World Federation of Parasitologists.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

