Microgravity-induced stress mechanisms in human stem cell-derived cardiomyocytes
- PMID: 35789849
- PMCID: PMC9249673
- DOI: 10.1016/j.isci.2022.104577
Microgravity-induced stress mechanisms in human stem cell-derived cardiomyocytes
Abstract
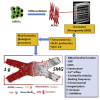
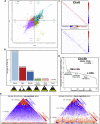
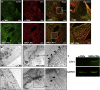
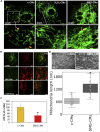
Exposure to outer space microgravity poses a risk for the development of various pathologies including cardiovascular disease. To study this, we derived cardiomyocytes (CMs) from human-induced pluripotent stem cells and exposed them to simulated microgravity (SMG). We combined different "omics" and chromosome conformation capture technologies with live-cell imaging of various transgenic lines to discover that SMG impacts on the contractile velocity and function of CMs via the induction of senescence processes. This is linked to SMG-induced changes of reactive oxygen species (ROS) generation and energy metabolism by mitochondria. Taken together, we uncover a microgravity-controlled axis causing contractile dysfunctions to CMs. Our findings can contribute to the design of preventive and therapeutic strategies against senescence-associated disease.
Keywords: Biological sciences; Cell biology; Molecular biology; Stem cells research.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Acharya A., Nemade H., Rajendra Prasad K., Khan K., Hescheler J., Blackburn N., Hemmersbach R., Papadopoulos S., Sachinidis A. Live-cell imaging of the contractile velocity and transient intracellular Ca(2+) fluctuations in human stem cell-derived cardiomyocytes. Cells. 2022;11:1280. doi: 10.3390/cells11081280. - DOI - PMC - PubMed
-
- Afshinnekoo E., Scott R.T., MacKay M.J., Pariset E., Cekanaviciute E., Barker R., Gilroy S., Hassane D., Smith S.M., Zwart S.R., et al. Fundamental biological features of spaceflight: advancing the field to enable deep-space exploration. Cell. 2020;183:1162–1184. doi: 10.1016/j.cell.2020.10.050. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

