Role of pericytes in blood-brain barrier preservation during ischemia through tunneling nanotubes
- PMID: 35790716
- PMCID: PMC9256725
- DOI: 10.1038/s41419-022-05025-y
Role of pericytes in blood-brain barrier preservation during ischemia through tunneling nanotubes
Abstract
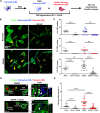
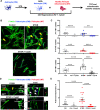
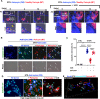
Crosstalk mechanisms between pericytes, endothelial cells, and astrocytes preserve integrity and function of the blood-brain-barrier (BBB) under physiological conditions. Long intercellular channels allowing the transfer of small molecules and organelles between distant cells called tunneling nanotubes (TNT) represent a potential substrate for energy and matter exchanges between the tripartite cellular compartments of the BBB. However, the role of TNT across BBB cells under physiological conditions and in the course of BBB dysfunction is unknown. In this work, we analyzed the TNT's role in the functional dialog between human brain endothelial cells, and brain pericytes co-cultured with human astrocytes under normal conditions or after exposure to ischemia/reperfusion, a condition in which BBB breakdown occurs, and pericytes participate in the BBB repair. Using live time-lapse fluorescence microscopy and laser-scanning confocal microscopy, we found that astrocytes form long TNT with pericytes and endothelial cells and receive functional mitochondria from both cell types through this mechanism. The mitochondrial transfer also occurred in multicellular assembloids of human BBB that reproduce the three-dimensional architecture of the BBB. Under conditions of ischemia/reperfusion, TNT formation is upregulated, and astrocytes exposed to oxygen-glucose deprivation were rescued from apoptosis by healthy pericytes through TNT-mediated transfer of functional mitochondria, an effect that was virtually abolished in the presence of TNT-destroying drugs. The results establish a functional role of TNT in the crosstalk between BBB cells and demonstrate that TNT-mediated mitochondrial transfer from pericytes rescues astrocytes from ischemia/reperfusion-induced apoptosis. Our data confirm that the pericytes might play a pivotal role in preserving the structural and functional integrity of BBB under physiological conditions and participate in BBB repair in brain diseases.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Shimizu F, Sano Y, Tominaga O, Maeda T, Abe M-A, Kanda T. Advanced glycation end-products disrupt the blood–brain barrier by stimulating the release of transforming growth factor–β by pericytes and vascular endothelial growth factor and matrix metalloproteinase–2 by endothelial cells in vitro. Neurobiol Aging. 2013;34:1902–12. doi: 10.1016/j.neurobiolaging.2013.01.012. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

