Avidity in antibody effector functions and biotherapeutic drug design
- PMID: 35790857
- PMCID: PMC9255845
- DOI: 10.1038/s41573-022-00501-8
Avidity in antibody effector functions and biotherapeutic drug design
Abstract
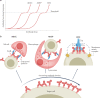
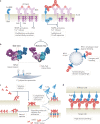
Antibodies are the cardinal effector molecules of the immune system and are being leveraged with enormous success as biotherapeutic drugs. A key part of the adaptive immune response is the production of an epitope-diverse, polyclonal antibody mixture that is capable of neutralizing invading pathogens or disease-causing molecules through binding interference and by mediating humoral and cellular effector functions. Avidity - the accumulated binding strength derived from the affinities of multiple individual non-covalent interactions - is fundamental to virtually all aspects of antibody biology, including antibody-antigen binding, clonal selection and effector functions. The manipulation of antibody avidity has since emerged as an important design principle for enhancing or engineering novel properties in antibody biotherapeutics. In this Review, we describe the multiple levels of avidity interactions that trigger the overall efficacy and control of functional responses in both natural antibody biology and their therapeutic applications. Within this framework, we comprehensively review therapeutic antibody mechanisms of action, with particular emphasis on engineered optimizations and platforms. Overall, we describe how affinity and avidity tuning of engineered antibody formats are enabling a new wave of differentiated antibody drugs with tailored properties and novel functions, promising improved treatment options for a wide variety of diseases.
© 2022. Springer Nature Limited.
Conflict of interest statement
J.S. and S.C.O. are employees of Genmab (a biotechnology company developing therapeutic antibodies including bispecific and avidity-engineered molecules), own Genmab warrants and/or stock and are inventors on patent applications relating to bispecific and avidity-engineered molecules assigned to Genmab. J.S. is a board member of the Antibody Society. G.A.L. is an employee of Genentech, a member of the Roche Group that develops and commercializes therapeutics including antibody-based drugs. P.W.H.I.P. is an employee of Lava Therapeutics and Leiden University Medical Center and a former employee of Genmab, owns options and/or stock of Lava Therapeutics and is an inventor on applications relating to bispecific and avidity-engineered molecules assigned to Lava Therapeutics and Genmab. He is a board member of The Antibody Society and provides consulting services on behalf of Sparring Bioconsult and served as an expert witness in a case of Roche versus Takeda. He is also an operational partner at Gilde Healthcare.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

