Effectiveness and cost-effectiveness of an intervention to improve Initial Medication Adherence to treatments for cardiovascular diseases and diabetes in primary care: study protocol for a pragmatic cluster randomised controlled trial and economic model (the IMA-cRCT study)
- PMID: 35790915
- PMCID: PMC9255541
- DOI: 10.1186/s12875-022-01727-6
Effectiveness and cost-effectiveness of an intervention to improve Initial Medication Adherence to treatments for cardiovascular diseases and diabetes in primary care: study protocol for a pragmatic cluster randomised controlled trial and economic model (the IMA-cRCT study)
Erratum in
-
Correction: Effectiveness and cost‑effectiveness of an intervention to improve Initial Medication Adherence to treatments for cardiovascular diseases and diabetes in primary care: study protocol for a pragmatic cluster randomised controlled trial and economic model (the IMA‑cRCT study).BMC Prim Care. 2024 Dec 27;25(1):437. doi: 10.1186/s12875-024-02694-w. BMC Prim Care. 2024. PMID: 39731006 Free PMC article. No abstract available.
Abstract
Background: Between 2 and 43% of patients who receive a new prescription in PC do not initiate their treatments. Non-initiation is associated with poorer clinical outcomes, more sick leave and higher costs to the healthcare system. Existing evidence suggests that shared decision-making positively impacts medication initiation. The IMA-cRCT assesses the effectiveness of the IMA intervention in improving adherence and clinical parameters compared to usual care in patients with a new treatment for cardiovascular disease and diabetes prescribed in PC, and its cost-effectiveness, through a cRCT and economic modelling.
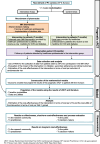
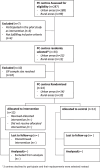
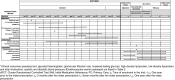
Methods: The IMA intervention is a shared decision-making intervention based on the Theoretical Model of Non-initiation. A cRCT will be conducted in 24 PC teams in Catalonia (Spain), randomly assigned to the intervention group (1:1), and community pharmacies in the catchment areas of the intervention PC teams. Healthcare professionals in the intervention group will apply the intervention to all patients who receive a new prescription for cardiovascular disease or diabetes treatment (no other prescription from the same pharmacological group in the previous 6 months). All the study variables will be collected from real-world databases for the 12 months before and after receiving a new prescription. Effectiveness analyses will assess impact on initiation, secondary adherence, cardiovascular risk, clinical parameters and cardiovascular events. Cost-effectiveness analyses will be conducted as part of the cRCT from a healthcare and societal perspective in terms of extra cost per cardiovascular risk reduction and improved adherence; all analyses will be clustered. Economic models will be built to assess the long-term cost-effectiveness of the IMA intervention, in terms of extra cost for gains in QALY and life expectancy, using clinical trial data and data from previous studies.
Discussion: The IMA-cRCT represents an innovative approach to the design and evaluation of behavioural interventions that use the principles of complex interventions, pragmatic trials and implementation research. This study will provide evidence on the IMA intervention and on a new methodology for developing and evaluating complex interventions. The results of the study will be disseminated among stakeholders to facilitate its transferability to clinical practice.
Trial registration: ClinicalTrials.gov, NCT05026775 . Registered 30th August 2021.
Keywords: Cardiovascular disease; Complex intervention; Cost-effectiveness analysis; Economic model; Medication adherence; Primary care; Shared decision-making.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- World Health Organization. Adherence to long-term therapies: evidence for action [Internet]. Geneva: World Health Organization; 2003. (https://apps.who.int/iris/bitstream/handle/10665/42682/9241545992.pdf?se...).
-
- DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40(9):794–811. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical