Integrative analysis of miRNA and mRNA profiles reveals that gga-miR-106-5p inhibits adipogenesis by targeting the KLF15 gene in chickens
- PMID: 35791010
- PMCID: PMC9258119
- DOI: 10.1186/s40104-022-00727-x
Integrative analysis of miRNA and mRNA profiles reveals that gga-miR-106-5p inhibits adipogenesis by targeting the KLF15 gene in chickens
Abstract
Background: Excessive abdominal fat deposition in commercial broilers presents an obstacle to profitable meat quality, feed utilization, and reproduction. Abdominal fat deposition depends on the proliferation of preadipocytes and their maturation into adipocytes, which involves a cascade of regulatory molecules. Accumulating evidence has shown that microRNAs (miRNAs) serve as post-transcriptional regulators of adipogenic differentiation in mammals. However, the miRNA-mediated molecular mechanisms underlying abdominal fat deposition in chickens are still poorly understood. This study aimed to investigate the biological functions and regulatory mechanism of miRNAs in chicken abdominal adipogenesis.
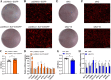
Results: We established a chicken model of abdominal adipocyte differentiation and analyzed miRNA and mRNA expression in abdominal adipocytes at different stages of differentiation (0, 12, 48, 72, and 120 h). A total of 217 differentially expressed miRNAs (DE-miRNAs) and 3520 differentially expressed genes were identified. Target prediction of DE-miRNAs and functional enrichment analysis revealed that the differentially expressed targets were significantly enriched in lipid metabolism-related signaling pathways, including the PPAR signaling and MAPK signaling pathways. A candidate miRNA, gga-miR-106-5p, exhibited decreased expression during the proliferation and differentiation of abdominal preadipocytes and was downregulated in the abdominal adipose tissues of fat chickens compared to that of lean chickens. gga-miR-106-5p was found to inhibit the proliferation and adipogenic differentiation of chicken abdominal preadipocytes. A dual-luciferase reporter assay suggested that the KLF15 gene, which encodes a transcriptional factor, is a direct target of gga-miR-106-5p. gga-miR-106-5p suppressed the post-transcriptional activity of KLF15, which is an activator of abdominal preadipocyte proliferation and differentiation, as determined with gain- and loss-of-function experiments.
Conclusions: gga-miR-106-5p functions as an inhibitor of abdominal adipogenesis by targeting the KLF15 gene in chickens. These findings not only improve our understanding of the specific functions of miRNAs in avian adipogenesis but also provide potential targets for the genetic improvement of excessive abdominal fat deposition in poultry.
Keywords: Abdominal fat; Adipogenesis; Chickens; KLF15; MiRNA; gga-miR-106-5p.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures










Similar articles
-
CircDOCK7 facilitates the proliferation and adipogenic differentiation of chicken abdominal preadipocytes through the gga-miR-301b-3p/ACSL1 axis.J Anim Sci Biotechnol. 2023 Jul 6;14(1):91. doi: 10.1186/s40104-023-00891-8. J Anim Sci Biotechnol. 2023. PMID: 37408086 Free PMC article.
-
Integrated Analysis of MiRNA and Genes Associated with Meat Quality Reveals that Gga-MiR-140-5p Affects Intramuscular Fat Deposition in Chickens.Cell Physiol Biochem. 2018;46(6):2421-2433. doi: 10.1159/000489649. Epub 2018 May 4. Cell Physiol Biochem. 2018. PMID: 29742492
-
Effects of immunosuppression-associated gga-miR-146a-5p on immune regulation in chicken macrophages by targeting the IRKA2 gene.Dev Comp Immunol. 2024 Jul;156:105159. doi: 10.1016/j.dci.2024.105159. Epub 2024 Mar 15. Dev Comp Immunol. 2024. PMID: 38492902
-
Adipogenic miRNA and meta-signature miRNAs involved in human adipocyte differentiation and obesity.Oncotarget. 2016 Jun 28;7(26):40830-40845. doi: 10.18632/oncotarget.8518. Oncotarget. 2016. PMID: 27049726 Free PMC article. Review.
-
Molecular Regulation of Lipogenesis, Adipogenesis and Fat Deposition in Chicken.Genes (Basel). 2021 Mar 13;12(3):414. doi: 10.3390/genes12030414. Genes (Basel). 2021. PMID: 33805667 Free PMC article. Review.
Cited by
-
Integrated Metabolomic and Transcriptomic Analysis Reveals Potential Gut-Liver Crosstalks in the Lipogenesis of Chicken.Animals (Basel). 2023 May 17;13(10):1659. doi: 10.3390/ani13101659. Animals (Basel). 2023. PMID: 37238090 Free PMC article.
-
Multi-Omics Insights into Regulatory Mechanisms Underlying Differential Deposition of Intramuscular and Abdominal Fat in Chickens.Biomolecules. 2025 Jan 15;15(1):134. doi: 10.3390/biom15010134. Biomolecules. 2025. PMID: 39858528 Free PMC article. Review.
-
CircDOCK7 facilitates the proliferation and adipogenic differentiation of chicken abdominal preadipocytes through the gga-miR-301b-3p/ACSL1 axis.J Anim Sci Biotechnol. 2023 Jul 6;14(1):91. doi: 10.1186/s40104-023-00891-8. J Anim Sci Biotechnol. 2023. PMID: 37408086 Free PMC article.
-
Non-Coding RNAs in Regulating Fat Deposition in Farm Animals.Animals (Basel). 2025 Mar 11;15(6):797. doi: 10.3390/ani15060797. Animals (Basel). 2025. PMID: 40150326 Free PMC article. Review.
-
Genome-Wide Identification, Evolution, and miRNA-22 Regulation of Kruppel-Like Factor (KLF) Gene Family in Chicken (Gallus gallus).Animals (Basel). 2024 Sep 6;14(17):2594. doi: 10.3390/ani14172594. Animals (Basel). 2024. PMID: 39272379 Free PMC article.
References
-
- Leclercq B, Blum JC, Boyer JP. Selecting broilers for low or high abdominal fat: initial observations. Br Poult Sci. 1980;21(2):107–113. doi: 10.1080/00071668008416644. - DOI
-
- Leclercq B. Genetic selection of meat-type chickens for high or low abdominal fat content. In: Leclercq B, Whitehead CC, editors. Leanness in domestic birds: genetic, metabolic and hormonal aspects. England: Butterworths; 1988. pp. 25–40.
Grants and funding
LinkOut - more resources
Full Text Sources

