Describing the population experiencing COVID-19 vaccine breakthrough following second vaccination in England: a cohort study from OpenSAFELY
- PMID: 35791013
- PMCID: PMC9255436
- DOI: 10.1186/s12916-022-02422-0
Describing the population experiencing COVID-19 vaccine breakthrough following second vaccination in England: a cohort study from OpenSAFELY
Abstract
Background: While the vaccines against COVID-19 are highly effective, COVID-19 vaccine breakthrough is possible despite being fully vaccinated. With SARS-CoV-2 variants still circulating, describing the characteristics of individuals who have experienced COVID-19 vaccine breakthroughs could be hugely important in helping to determine who may be at greatest risk.
Methods: With the approval of NHS England, we conducted a retrospective cohort study using routine clinical data from the OpenSAFELY-TPP database of fully vaccinated individuals, linked to secondary care and death registry data and described the characteristics of those experiencing COVID-19 vaccine breakthroughs.
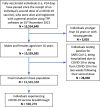
Results: As of 1st November 2021, a total of 15,501,550 individuals were identified as being fully vaccinated against COVID-19, with a median follow-up time of 149 days (IQR: 107-179). From within this population, a total of 579,780 (<4%) individuals reported a positive SARS-CoV-2 test. For every 1000 years of patient follow-up time, the corresponding incidence rate (IR) was 98.06 (95% CI 97.93-98.19). There were 28,580 COVID-19-related hospital admissions, 1980 COVID-19-related critical care admissions and 6435 COVID-19-related deaths; corresponding IRs 4.77 (95% CI 4.74-4.80), 0.33 (95% CI 0.32-0.34) and 1.07 (95% CI 1.06-1.09), respectively. The highest rates of breakthrough COVID-19 were seen in those in care homes and in patients with chronic kidney disease, dialysis, transplant, haematological malignancy or who were immunocompromised.
Conclusions: While the majority of COVID-19 vaccine breakthrough cases in England were mild, some differences in rates of breakthrough cases have been identified in several clinical groups. While it is important to note that these findings are simply descriptive and cannot be used to answer why certain groups have higher rates of COVID-19 breakthrough than others, the emergence of the Omicron variant of COVID-19 coupled with the number of positive SARS-CoV-2 tests still occurring is concerning and as numbers of fully vaccinated (and boosted) individuals increases and as follow-up time lengthens, so too will the number of COVID-19 breakthrough cases. Additional analyses, to assess vaccine waning and rates of breakthrough COVID-19 between different variants, aimed at identifying individuals at higher risk, are needed.
Keywords: COVID-19; EHR data; Vaccine breakthrough.
© 2022. The Author(s).
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form and declare the following: BG has received research funding from Health Data Research UK (HDRUK), the Laura and John Arnold Foundation, the Wellcome Trust, the NIHR Oxford Biomedical Research Centre, the NHS National Institute for Health Research School of Primary Care Research, the Mohn-Westlake Foundation, the Good Thinking Foundation, the Health Foundation, and the World Health Organisation; he also receives personal income from speaking and writing for lay audiences on the misuse of science. IJD holds shares in GlaxoSmithKline (GSK).
Figures
References
-
- COVID-19: the green book, chapter 14a. GOV.UK. 2020. https://www.gov.uk/government/publications/covid-19-the-green-book-chapt.... Accessed 20 Oct 2021.
-
- Dosing information COVID-19 primary vaccination. SPS - Specialist Pharmacy Service. 2021. https://www.sps.nhs.uk/articles/dosing-information-covid-19-primary-vacc.... Accessed 1 Mar 2022.
-
- Coronavirus (COVID-19) in the UK. GOV.UK. https://coronavirus.data.gov.uk/details/vaccinations. Accessed 9 Sep 2021.
-
- Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous