Cytokine- and chemokine-induced inflammatory colorectal tumor microenvironment: Emerging avenue for targeted therapy
- PMID: 35791509
- PMCID: PMC9395317
- DOI: 10.1002/cac2.12295
Cytokine- and chemokine-induced inflammatory colorectal tumor microenvironment: Emerging avenue for targeted therapy
Abstract
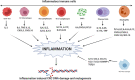
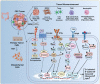
Colorectal cancer (CRC) is a predominant life-threatening cancer, with liver and peritoneal metastases as the primary causes of death. Intestinal inflammation, a known CRC risk factor, nurtures a local inflammatory environment enriched with tumor cells, endothelial cells, immune cells, cancer-associated fibroblasts, immunosuppressive cells, and secretory growth factors. The complex interactions of aberrantly expressed cytokines, chemokines, growth factors, and matrix-remodeling enzymes promote CRC pathogenesis and evoke systemic responses that affect disease outcomes. Mounting evidence suggests that these cytokines and chemokines play a role in the progression of CRC through immunosuppression and modulation of the tumor microenvironment, which is partly achieved by the recruitment of immunosuppressive cells. These cells impart features such as cancer stem cell-like properties, drug resistance, invasion, and formation of the premetastatic niche in distant organs, promoting metastasis and aggressive CRC growth. A deeper understanding of the cytokine- and chemokine-mediated signaling networks that link tumor progression and metastasis will provide insights into the mechanistic details of disease aggressiveness and facilitate the development of novel therapeutics for CRC. Here, we summarized the current knowledge of cytokine- and chemokine-mediated crosstalk in the inflammatory tumor microenvironment, which drives immunosuppression, resistance to therapeutics, and metastasis during CRC progression. We also outlined the potential of this crosstalk as a novel therapeutic target for CRC. The major cytokine/chemokine pathways involved in cancer immunotherapy are also discussed in this review.
Keywords: chemokine; colorectal cancer; cytokine; drug resistance; epithelial-mesenchymal transition; immunosuppression; immunotherapy; inflammation; metastasis; tumor microenvironment.
© 2022 Sidra Medicine. Cancer Communications published by John Wiley & Sons Australia, Ltd on behalf of Sun Yat-Sen University Cancer Center.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Van Cutsem E, Oliveira J. Advanced colorectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow‐up. Ann Oncol. 2009;20(Suppl 4):61–3. - PubMed
-
- Biasco G, Derenzini E, Grazi G, Ercolani G, Ravaioli M, Pantaleo MA, et al. Treatment of hepatic metastases from colorectal cancer: many doubts, some certainties. Cancer Treat Rev. 2006;32(3):214–28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

