Lanthionine Ketimine Ethyl Ester Accelerates Remyelination in a Mouse Model of Multiple Sclerosis
- PMID: 35791633
- PMCID: PMC9272172
- DOI: 10.1177/17590914221112352
Lanthionine Ketimine Ethyl Ester Accelerates Remyelination in a Mouse Model of Multiple Sclerosis
Abstract
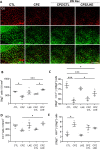
Although over 20 disease modifying therapies are approved to treat Multiple Sclerosis (MS), these do not increase remyelination of demyelinated axons or mitigate axon damage. Previous studies showed that lanthionine ketenamine ethyl ester (LKE) reduces clinical signs in the experimental autoimmune encephalomyelitis (EAE) mouse model of MS and increased maturation of oligodendrocyte (OL) progenitor cells (OPCs) in vitro. In the current study, we used the cuprizone (CPZ) demyelination model of MS to test if LKE could increase remyelination. The corpus callosum (CC) and somatosensory cortex was examined by immunohistochemistry (IHC), electron microscopy and for mRNA expression changes in mice provided 5 weeks of CPZ diet followed by 2 weeks of normal diet in the presence of LKE or vehicle. A significant increase in the number of myelinated axons, and increased myelin thickness was observed in the CC of LKE-treated groups compared to vehicle-treated groups. LKE also increased myelin basic protein and proteolipid protein expression in the CC and cortex, and increased the number of mature OLs in the cortex. In contrast, LKE did not increase the percentage of proliferating OPCs suggesting effects on OPC survival and differentiation but not proliferation. The effects of LKE on OL maturation and remyelination were supported by similar changes in their relative mRNA levels. Interestingly, LKE did not have significant effects on GFAP or Iba1 immunostaining or mRNA levels. These findings suggest that remyelinating actions of LKE can potentially be formulated to induce remyelination in neurological diseases associated with demyelination including MS.
Keywords: demyelination < NEURO degeneration; myelination < NEURO development; oligodendrocytes < NEURO glia; regeneration < NEURO repair.
Conflict of interest statement
Figures








References
-
- Aryanpour R., Zibara K., Pasbakhsh P., Namjoo S. B., Ghanbari Z., Mahmoudi A., Amani R., Kashani S. (2021). 17β- Estradiol reduces demyelination in cuprizone-fed mice by promoting M2 microglia polarity and regulating NLRP3 inflammasome. Neuroscience, 463, 116–127. https://doi.org/10.1016/j.neuroscience.2021.03.025. - PubMed
-
- Brittain J. M., Pan R., You H., Brustovetsky T., Brustovetsky N., Zamponi G. W., Lee W. H., Khanna R. (2012). Disruption of nmdar-crmp-2 signaling protects against focal cerebral ischemic damage in the rat middle cerebral artery occlusion model. Channels (Austin), 6(1), 52–59. 10.4161/chan.18919 - DOI - PMC - PubMed
-
- Brittain J. M., Piekarz A. D., Wang Y., Kondo T., Cummins T. R., Khanna R. (2009). An atypical role for collapsin response mediator protein 2 (crmp-2) in neurotransmitter release via interaction with presynaptic voltage-gated calcium channels. Journal of Biological Chemistry, 284(45), 31375–31390. 10.1074/jbc.M109.009951 - DOI - PMC - PubMed
-
- Brustovetsky T., Pellman J. J., Yang X. F., Khanna R., Brustovetsky N. (2014). Collapsin response mediator protein 2 (crmp2) interacts with n-methyl-d-aspartate (nmda) receptor and na + /ca2 + exchanger and regulates their functional activity. Journal of Biological Chemistry, 289(11), 7470–7482. 10.1074/jbc.M113.518472 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
