Efficacy and safety of mirikizumab in psoriasis: results from a 52-week, double-blind, placebo-controlled, randomized withdrawal, phase III trial (OASIS-1)
- PMID: 35791755
- PMCID: PMC10087045
- DOI: 10.1111/bjd.21743
Efficacy and safety of mirikizumab in psoriasis: results from a 52-week, double-blind, placebo-controlled, randomized withdrawal, phase III trial (OASIS-1)
Abstract
Background: Interleukin-23 inhibitors are effective and safe for treating moderate-to-severe plaque psoriasis.
Objectives: To evaluate the efficacy and safety of mirikizumab in adult patients with moderate-to-severe plaque psoriasis through 52 weeks in a phase III randomized controlled trial.
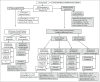
Methods: OASIS-1 (NCT03482011) was a double-blind, placebo-controlled, randomized withdrawal, phase III trial. Patients (n = 530, randomized 4 : 1) received subcutaneous mirikizumab 250 mg or placebo every 4 weeks (Q4W) through week 16. Coprimary endpoints were superiority of mirikizumab vs. placebo on static Physician's Global Assessment (sPGA; score of 0 or 1 with ≥ 2-point improvement) and ≥ 90% improvement in Psoriasis Area and Severity Index (PASI 90, responders) at week 16. Mirikizumab responders were rerandomized (1 : 1 : 1) to mirikizumab 250 mg every 8 weeks (Q8W), mirikizumab 125 mg Q8W, or placebo Q8W through week 52. Secondary endpoints were evaluated at weeks 16 and 52. Safety was monitored in all patients.
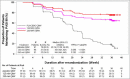
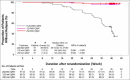
Results: All primary and key secondary endpoints were met. At week 16, sPGA(0,1) responses were significantly greater with mirikizumab (293 of 423, 69·3%) than placebo (seven of 107, 6·5%) (P < 0·001). PASI 90 response was also greater with mirikizumab (272 of 423, 64·3%) than placebo (seven of 107, 6·5%) (P < 0·001). Significantly more patients in the mirikizumab arms achieved PASI 75 and PASI 100 (mirikizumab 349, 82·5% and 137, 32·4%; placebo 10, 9·3% and 1, 0·9%, respectively; all P < 0·001). At week 52, PASI 90, PASI 100 and sPGA(0,1) responses were mirikizumab 250Q4W/placeboQ8W (N = 91; 19%, 10%, 18%), mirikizumab 250Q4W/125Q8W (N = 90; 86%, 59%, 86%) and mirikizumab 250Q4W/250Q8W (N = 91; 86%, 60%, 82%; all P < 0·001), respectively. Rates of serious adverse events were similar across treatments (induction: mirikizumab 1·2% vs. placebo 1·9%; maintenance: mirikizumab 250Q4W/125Q8W 1%, mirikizumab 250Q4W/250Q8W 3% vs. placebo 3%). No deaths occurred.
Conclusions: Mirikizumab was superior to placebo at week 16 and maintained efficacy through week 52, with no new safety signals. What is already known about this topic? Interleukin (IL)-23 is a key cytokine in the pathogenesis of psoriasis. Drugs targeting the p19 subunit of IL-23 have recently been approved for the treatment of adult patients with moderate-to-severe plaque psoriasis. Patients with moderate-to-severe plaque psoriasis achieved significantly greater improvements in skin measures and patient-reported quality-of-life measures after 16 weeks when treated every 8 weeks with mirikizumab compared with placebo in a phase II clinical trial. What does this study add? Compared with placebo, mirikizumab demonstrated high levels of efficacy at week 16 in a large phase III trial; safety profiles were similar between the mirikizumab and placebo arms. After week 16, patients maintained on doses of mirikizumab 250 mg every 8 weeks (Q8W) or 125 mg Q8W showed similar efficacy and favourable safety profiles over 52 weeks, whereas patients switched to placebo gradually lost efficacy over time.
© 2022 The Authors. British Journal of Dermatology published by John Wiley & Sons Ltd on behalf of British Association of Dermatologists.
Conflict of interest statement
A.B. has served as a scientific adviser and/or clinical study investigator for AbbVie, Abcentra, Aligos, Almirall, Amgen, Arcutis, Arena, Aslan, Athenex, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, EcoR1, Eli Lilly and Company, Evommune, Forte, Galderma, Incyte, Janssen, Landos, LEO, Novartis, Pfizer, Rapt, Regeneron, Sanofi Genzyme, Sun Pharma, UCB Pharma and Vibliome. A.B.K. is a consultant and investigator for AbbVie, Janssen, Eli Lilly, Novartis, UCB and Target RWE; is an investigator for Bristol Meyers Squibb; is a consultant for Amgen, LEO, Meiji Seiki Pharma, Ventyx and Moonlake; has received fellowship funding from Janssen and AbbVie; has served previously on the board of directors and was past president of the International Psoriasis Council; and has served on the board of directors of Almirall. M.A. has served as a consultant, lecturer and researcher for, and/or has received research grants from companies manufacturing drugs for psoriasis, including AbbVie, Almirall, Amgen, Bayer, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Centocor, Dermira, Eli Lilly, Genzyme, Hexal, Incyte, Janssen, LEO, Medac, MSD, Mylan B.V., Novartis, Pfizer, Regeneron, Sandoz and UCB. Y.O. reports grants from Sun Pharma Japan Ltd, Maruho Co, Ltd, Eisai Co, Ltd and Torii Pharmaceutical Co, Ltd; has received consulting fees from Boehringer Ingelheim, UCB Japan Co, Ltd, and Eli Lilly Japan KK; has received payment for lectures from Kyowa Kirin Co, Ltd, Novartis Pharma KK, Eli Lilly Japan KK, AbbVie GK, UCB Japan Co, Ltd, Janssen Pharmaceutical KK, Taiho Pharmaceutical Co, Ltd, Maruho Co, Ltd and Amgen Inc.; and has participated on a data safety monitoring board or advisory board for Boehringer Ingelheim, UCB Japan Co, Ltd and Eli Lilly Japan KK. M.M.W., C.R.C, A.S., V.A. and O.O. are a full‐time employees and stockholders of Eli Lilly and Company. B.S. has served as a consultant (honoraria) for AbbVie, Almirall, Amgen, Arcutis, Arena, Aristea, Asana, Boehringer Ingelheim, Immunic Therapeutics, Bristol Myers Squibb, Connect Biopharma, Dermavant, Eli Lilly, EPI Health, Evelo Biosciences, Janssen, LEO, Maruho, Meiji Seika Pharma, Mindera Health, Novartis, Ono, Pfizer, UCB Pharma, Sun Pharma, Regeneron, Sanofi Genzyme, Union Therapeutics, Ventyxbio and vTv Therapeutics; is a stockholder of Connect Biopharma and Mindera Health; has served as a speaker for AbbVie, Eli Lilly, Janssen, Regeneron and Sanofi Genzyme; has been a co‐scientific director (consulting fee) for CorEvitas (formerly Corrona) Psoriasis Registry; has been an investigator for Dermavant, AbbVie, CorEvitas Psoriasis Registry, Dermira, Cara and Novartis; and has been editor in chief (honorarium) of the
Figures


Comment in
-
Psoriasis treatment: no more room on the summit?Br J Dermatol. 2022 Dec;187(6):837. doi: 10.1111/bjd.21848. Epub 2022 Sep 5. Br J Dermatol. 2022. PMID: 36065496 No abstract available.
References
-
- Parisi R, Symmons DP, Griffiths CE et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013; 133:377–85. - PubMed
-
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet 2007; 370:263–71. - PubMed
-
- Reich K, Armstrong AW, Foley P et al. Efficacy and safety of guselkumab, an anti‐interleukin‐23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double‐blind, placebo‐ and active comparator‐controlled VOYAGE 2 trial. J Am Acad Dermatol 2017; 76:418–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

