Development of a humanized HLA-A30 transgenic mouse model
- PMID: 35791899
- PMCID: PMC9434587
- DOI: 10.1002/ame2.12225
Development of a humanized HLA-A30 transgenic mouse model
Abstract
Background: There are remarkable genetic differences between animal major histocompatibility complex (MHC) systems and the human leukocyte antigen (HLA) system. HLA transgenic humanized mouse model systems offer a much better method to study the HLA-A-related principal mechanisms for vaccine development and HLA-A-restricted responses against infection in human.
Methods: A recombinant gene encoding the chimeric HLA-A30 monochain was constructed. This HHD molecule contains the following: α1-α2 domains of HLA-A30, α3 and cytoplasmic domains of H-2Db , linked at its N-terminus to the C-terminus of human β2m by a 15-amino-acid peptide linker. The recombinant gene encoding the chimeric HLA-A30 monochain cassette was introduced into bacterial artificial chromosome (BAC) CH502-67J3 containing the HLA-A01 gene locus by Red-mediated homologous recombination. Modified BAC CH502-67J3 was microinjected into the pronuclei of wild-type mouse oocytes. This humanized mouse model was further used to assess the immune responses against influenza A virus (H1N1) pdm09 clinically isolated from human patients. Immune cell population, cytokine production, and histopathology in the lung were analyzed.
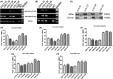
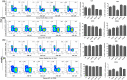
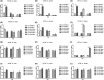
Results: We describe a novel human β2m-HLA-A30 (α1α2)-H-2Db (α3 transmembrane cytoplasmic) (HHD) monochain transgenic mouse strain, which contains the intact HLA-A01 gene locus including 49 kb 5'-UTR and 74 kb 3'-UTR of HLA-A01*01. Five transgenic lines integrated into the large genomic region of HLA-A gene locus were obtained, and the robust expression of exogenous transgene was detected in various tissues from A30-18# and A30-19# lines encompassing the intact flanking sequences. Flow cytometry revealed that the introduction of a large genomic region in HLA-A gene locus can influence the immune cell constitution in humanized mice. Pdm09 infection caused a similar immune response among HLA-A30 Tg humanized mice and wild-type mice, and induced the rapid increase of cytokines, including IFN-γ, TNF-α, and IL-6, in both HLA-A30 humanized Tg mice and wild-type mice. The expression of HLA-A30 transgene was dramatically promoted in tissues from A30-9# line at 3 days post-infection (dpi).
Conclusions: We established a promising preclinical research animal model of HLA-A30 Tg humanized mouse, which could accelerate the identification of novel HLA-A30-restricted epitopes and vaccine development, and support the study of HLA-A-restricted responses against infection in humans.
Keywords: HLA-A30; humanized mouse; immunology; major histocompatibility complex (MHC).
© 2022 The Authors. Animal Models and Experimental Medicine published by John Wiley & Sons Australia, Ltd on behalf of The Chinese Association for Laboratory Animal Sciences.
Conflict of interest statement
The authors declare that they have no conflicts of interest relevant to this work. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Xiao‐hui Zhou is an Editorial Board member of AMEM and a co‐author of this article. To minimize bias, he was excluded from all editorial decision‐making related to the acceptance of this article for publication.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

