Taste quality and hunger interactions in a feeding sensorimotor circuit
- PMID: 35791902
- PMCID: PMC9292995
- DOI: 10.7554/eLife.79887
Taste quality and hunger interactions in a feeding sensorimotor circuit
Abstract
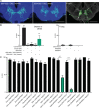
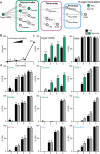
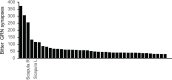
Taste detection and hunger state dynamically regulate the decision to initiate feeding. To study how context-appropriate feeding decisions are generated, we combined synaptic resolution circuit reconstruction with targeted genetic access to specific neurons to elucidate a gustatory sensorimotor circuit for feeding initiation in adult Drosophila melanogaster. This circuit connects gustatory sensory neurons to proboscis motor neurons through three intermediate layers. Most neurons in this pathway are necessary and sufficient for proboscis extension, a feeding initiation behavior, and respond selectively to sugar taste detection. Pathway activity is amplified by hunger signals that act at select second-order neurons to promote feeding initiation in food-deprived animals. In contrast, the feeding initiation circuit is inhibited by a bitter taste pathway that impinges on premotor neurons, illuminating a local motif that weighs sugar and bitter taste detection to adjust the behavioral outcomes. Together, these studies reveal central mechanisms for the integration of external taste detection and internal nutritive state to flexibly execute a critical feeding decision.
Keywords: D. melanogaster; circuits; feeding; gustatory; neuroscience; sensorimotor; taste.
© 2022, Shiu, Sterne et al.
Conflict of interest statement
PS, GS, SE, BD, KS No competing interests declared
Figures







Update of
References
-
- Bates AS, Schlegel P, Roberts RJV, Drummond N, Tamimi IFM, Turnbull R, Zhao X, Marin EC, Popovici PD, Dhawan S, Jamasb A, Javier A, Serratosa Capdevila L, Li F, Rubin GM, Waddell S, Bock DD, Costa M, Jefferis G. Complete connectomic reconstruction of olfactory projection neurons in the fly brain. Current Biology. 2020b;30:3183–3199. doi: 10.1016/j.cub.2020.06.042. - DOI - PMC - PubMed
-
- Buhmann J, Sheridan A, Malin-Mayor C, Schlegel P, Gerhard S, Kazimiers T, Krause R, Nguyen TM, Heinrich L, Lee WCA, Wilson R, Saalfeld S, Jefferis G, Bock DD, Turaga SC, Cook M, Funke J. Automatic detection of synaptic partners in a whole-brain Drosophila electron microscopy data set. Nature Methods. 2021;18:771–774. doi: 10.1038/s41592-021-01183-7. - DOI - PMC - PubMed
-
- Bushey D. CircuitCatcher. GitHub. 2019 https://github.com/DanBushey/CircuitCatcher
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

