Modulation of the Itaconate Pathway Attenuates Murine Lupus
- PMID: 35791960
- PMCID: PMC11337117
- DOI: 10.1002/art.42284
Modulation of the Itaconate Pathway Attenuates Murine Lupus
Abstract
Objective: Itaconic acid, a Krebs cycle-derived immunometabolite, is synthesized by myeloid cells in response to danger signals to control inflammasome activation, type I interferon (IFN) responses, and oxidative stress. As these pathways are dysregulated in systemic lupus erythematosus (SLE), we investigated the role of an itaconic acid derivative in the treatment of established murine lupus.
Methods: Female (NZW × NZB)F1 lupus-prone mice were administered 4-octyl itaconate (4-OI) or vehicle starting after clinical onset of disease (30 weeks of age) for 4 weeks (n = 10 mice /group). At 34 weeks of age (peak disease activity), animals were euthanized, organs and serum were collected, and clinical, metabolic, and immunologic parameters were evaluated.
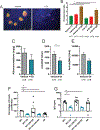
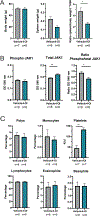
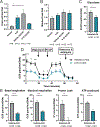
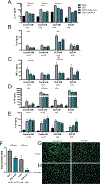
Results: Proteinuria, kidney immune complex deposition, renal scores of severity and inflammation, and anti-RNP autoantibodies were significantly reduced in the 4-OI treatment group compared to the vehicle group. Splenomegaly decreased in the 4-OI group compared to vehicle, with decreases in activation markers in innate and adaptive immune cells, increases in CD8+ T cell numbers, and inhibition of JAK1 activation. Gene expression analysis in splenocytes showed significant decreases in type I IFN and proinflammatory cytokine genes and increased Treg cell-associated markers in the 4-OI group compared to the vehicle group. In human control and lupus myeloid cells, 4-OI in vitro treatment decreased proinflammatory responses and B cell responses.
Conclusions: These results support targeting immunometabolism as a potentially viable approach in autoimmune disease treatment, with 4-OI displaying beneficial roles attenuating immune dysregulation and organ damage in lupus.
Published 2022. This article is a U.S. Government work and is in the public domain in the USA.
Conflict of interest statement
There are no conflicts of interest.
Figures


References
-
- Mohan C, Putterman C. Genetics and pathogenesis of systemic lupus erythematosus and lupus nephritis. Nat Rev Nephrol. 2015;11(6):329–41. - PubMed
-
- Kiriakikidou M, Chin KL. Systemic Lupus Erythematosus. Annals of Internal Medicine. 2020;172(11):ITC81–ITC96. - PubMed
-
- Crow MK, Olferiev M, Kirou KA. Type I Interferons in Autoimmune Disease. Annual Review of Pathology: Mechanisms of Disease. 2019;14(1):369–93. - PubMed
-
- Li Z, Guo J, Bi L. Role of the NLRP3 inflammasome in autoimmune diseases. Biomedicine & Pharmacotherapy. 2020;130:110542. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

