Evaluation of Dyspnea and Exercise Intolerance After Acute Pulmonary Embolism
- PMID: 35792185
- PMCID: PMC10107059
- DOI: 10.1016/j.chest.2022.06.036
Evaluation of Dyspnea and Exercise Intolerance After Acute Pulmonary Embolism
Abstract
Long-term dyspnea and exercise intolerance are common clinical problems after acute pulmonary embolism. Unfortunately, no single test can distinguish among the range of potential pathologic outcomes after pulmonary embolism. We illustrate a stepwise approach to post-pulmonary embolism evaluation that uses a hierarchic series of clinically validated diagnostic tests. The algorithm is represented by the acronym SEARCH, which stands for Symptom screening, Exercise testing, Arterial perfusion, Resting echocardiography, Confirmatory chest imaging, and Hemodynamics measured by right heart catheterization. We illustrate the algorithm with a patient whom we saw in our pulmonary embolism follow-up clinic. Patients are asked at least 6 months after pulmonary embolism whether they have returned to their baseline level of respiratory comfort and exercise tolerance. Patients with dyspnea and exercise intolerance undergo noninvasive cardiopulmonary exercise testing to identify elevated ventilatory dead space ratios, decreased stroke volume augmentation with exercise, and other physiologic abnormalities during exertion. Ventilation-perfusion scanning is performed on those patients with exercise-related physiologic findings to confirm the presence of residual pulmonary arterial obstruction or to suggest alternative diagnoses. Resting echocardiography may provide evidence of pulmonary hypertension; confirmatory imaging with pulmonary angiography or CT angiography may disclose findings characteristic of chronic pulmonary artery obstruction. Finally, right heart catheterization is performed to confirm chronic thromboembolic pulmonary hypertension; if resting pulmonary hemodynamics are normal, then invasive cardiopulmonary exercise testing may disclose exercise-induced defects.
Keywords: dyspnea; exercise intolerance; pulmonary embolism.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

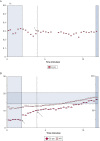

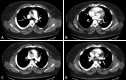
References
-
- Klok F.A., Tijmensen J.E., Haeck M.L., van Kralingen K.W., Huisman M.V. Persistent dyspnea complaints at long-term follow-up after an episode of acute pulmonary embolism: results of a questionnaire. Eur J Intern Med. 2008;19(8):625–629. - PubMed
-
- Klok F.A., van Kralingen K.W., van Dijk A.P., et al. Quality of life in long-term survivors of acute pulmonary embolism. Chest. 2010;138(6):1432–1440. - PubMed
-
- van Es J., den Exter P.L., Kaptein A.A., et al. Quality of life after pulmonary embolism as assessed with SF-36 and PEmb-QoL. Thromb Res. 2013;132(5):500–505. - PubMed
-
- Fernandes T.M., Alotaibi M., Strozza D.M., et al. Dyspnea postpulmonary embolism from physiological dead space proportion and stroke volume defects during exercise. Chest. 2020;157(4):936–944. - PubMed

