Unified, Asymmetric Total Synthesis of the Asnovolins and Related Spiromeroterpenoids: A Fragment Coupling Approach
- PMID: 35792599
- PMCID: PMC9667390
- DOI: 10.1021/jacs.2c05366
Unified, Asymmetric Total Synthesis of the Asnovolins and Related Spiromeroterpenoids: A Fragment Coupling Approach
Abstract
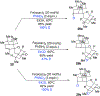
3,5-Dimethylorsellinic acid (DMOA)-derived spiromeroterpenoids are a unique natural product family with attractive structures, unconventional stereochemistry, and potent biological activities. Herein, we report the first asymmetric total syntheses of the asnovolins, DMOA-derived spiromeroterpenoids. The spirocyclic skeleton was efficiently assembled through a sterically hindered bis-neopentyl 1,2-addition coupling/oxidative Michael addition sequence. The unusual axial C12-methyl stereochemistry was established via metal hydrogen atom transfer (MHAT) reduction involving a chair-to-boat conformational change. The mechanism of the HAT process was studied through both deuterium labeling and computational studies. Attempted late-stage alkene isomerization of an exocyclic enone proved to be challenging and resulted in hetero-Diels-Alder dimerization, which ultimately led to development of an alternative desaturation/coupling sequence. Endgame core modifications including orthogonal desaturation, Sc(III)-promoted regioselective Baeyer-Villiger oxidation, and Meerwein-Ponndorf-Verley reduction enabled collective syntheses of five asnovolin-related natural products. This study demonstrates the utility of anionic fragment coupling to assemble a sterically congested molecular framework and provides a foundation for the synthesis of spiromeroterpenoid congeners with higher oxidation states for biological studies.
Figures




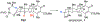


References
-
- Okuyama E; Yamazaki M Fumigatonin, a New Meroterpenoid from Aspergillus Fumigatus. Tetrahedron Lett. 1984, 25 (30), 3233–3234.
-
- Kusano M; Koshino H; Uzawa J; Fujioka S; Kawano T; Kimura Y Simplicissin, a New Pollen Growth Inhibitor Produced by the Fungus, Penicillium Cf. Simplidssimum (Oudemans) Thorn No. 410. Biosci. Biotechnol. Biochem 1997, 61 (12), 2153–2155. - PubMed
-
- Rank C; Phipps RK; Harris P; Fristrup P; Larsen TO; Gotfredsen CH Novofumigatonin, a New Orthoester Meroterpenoid from Aspergillus Novofumigatus. Org. Lett 2008, 10 (3), 401–404. - PubMed
-
- Liu H; Li X-M; Liu Y; Zhang P; Wang J-N; Wang B-G Chermesins A–D: Meroterpenoids with a Drimane-Type Spirosesquiterpene Skeleton from the Marine Algal-Derived Endophytic Fungus Penicillium Chermesinum EN-480. J. Nat. Prod 2016, 79 (4), 806–811. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

