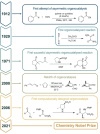
Theoretical Perspectives in Organocatalysis
- PMID: 35792702
- PMCID: PMC9804221
- DOI: 10.1002/chem.202201570
Theoretical Perspectives in Organocatalysis
Abstract
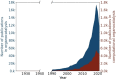
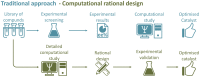
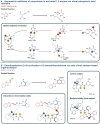
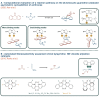
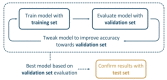
It is clear that the field of organocatalysis is continuously expanding during the last decades. With increasing computational capacity and new techniques, computational methods have provided a more economic approach to explore different chemical systems. This review offers a broad yet concise overview of current state-of-the-art studies that have employed novel strategies for catalyst design. The evolution of the all different theoretical approaches most commonly used within organocatalysis is discussed, from the traditional approach, manual-driven, to the most recent one, machine-driven.
Keywords: asymmetric organocatalysis; computational design; computationally-led catalyst design; organocatalysts; prediction.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Langenbeck W., Justus Liebigs Ann. Chem. 1929, 469, 16–25.
-
- Hajos Z. G., Parrish D. R., J. Org. Chem. 1974, 39, 1615–1621.
-
- None
-
- Ahrendt K. A., Borths C. J., MacMillan D. W. C., J. Am. Chem. Soc. 2000, 122, 4243–4244;
-
- List B., J. Am. Chem. Soc. 2002, 124, 5656–5657. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

