Neutralization of Omicron sublineages and Deltacron SARS-CoV-2 by three doses of BNT162b2 vaccine or BA.1 infection
- PMID: 35792746
- PMCID: PMC9331225
- DOI: 10.1080/22221751.2022.2099305
Neutralization of Omicron sublineages and Deltacron SARS-CoV-2 by three doses of BNT162b2 vaccine or BA.1 infection
Abstract
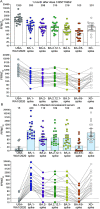
Distinct SARS-CoV-2 Omicron sublineages have evolved showing increased fitness and immune evasion than the original Omicron variant BA.1. Here, we report the neutralization activity of sera from BNT162b2 vaccinated individuals or unimmunized Omicron BA.1-infected individuals against Omicron sublineages and "Deltacron" variant (XD). BNT162b2 post-dose 3 immune sera neutralized USA-WA1/2020, Omicron BA.1-, BA.2-, BA.2.12.1-, BA.3-, BA.4/5-, and XD-spike SARS-CoV-2s with geometric mean titres (GMTs) of 1335, 393, 298, 315, 216, 103, and 301, respectively; thus, BA.4/5 SARS-CoV-2 spike variant showed the highest propensity to evade vaccine neutralization compared to the original Omicron variants BA.1. BA.1-convalescent sera neutralized USA-WA1/2020, BA.1-, BA.2-, BA.2.12.1-, BA.3-, BA.4/5-, and Deltacron-spike SARS-CoV-2s with GMTs of 15, 430, 110, 109, 102, 25, and 284, respectively. The unique mutation F486V in the BA.4/5 spike contributes to the increased evasion of antibody neutralization by sublineage BA.4/5. The low neutralization titres of vaccinated sera or convalescent sera from BA.1 infected individuals against the emerging and rapidly spreading Omicron BA.4/5 variants provide important results for consideration in the selection of an updated vaccine in the current Omicron wave.Trial registration: ClinicalTrials.gov; identifier: NCT04368728.
Keywords: BNT162b2 vaccine; Omicron; SARS-CoV-2; neutralization; variants.
Conflict of interest statement
X.X. and P.-Y.S. have filed a patent on the reverse genetic system of SARS-CoV-2. C.K., J.Z., H.X., X.X., and P.-Y.S. received compensation from Pfizer to perform the project. Q.Y., M.C., D.C., K.U.J., and K.A.S. are employees of Pfizer and may hold stock options. K.A.S. and Q.Y. are inventors on a patent application related to RNA-based COVID-19 vaccines. U.S. and A.M. are inventors on patents and patent applications related to RNA technology and COVID-19 vaccines. A.M is an employee of BioNTech and may hold stock options.
Figures
References
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
