Association of Prenatal Exposure to Endocrine-Disrupting Chemicals With Liver Injury in Children
- PMID: 35793087
- PMCID: PMC9260485
- DOI: 10.1001/jamanetworkopen.2022.20176
Association of Prenatal Exposure to Endocrine-Disrupting Chemicals With Liver Injury in Children
Abstract
Importance: Prenatal exposures to endocrine-disrupting chemicals (EDCs) may increase the risk for liver injury in children; however, human evidence is scarce, and previous studies have not considered potential EDC-mixture effects. Furthermore, the association between prenatal EDC exposure and hepatocellular apoptosis in children has not been studied previously.
Objective: To investigate associations of prenatal exposure to EDC mixtures with liver injury risk and hepatocellular apoptosis in childhood.
Design, setting, and participants: This prospective cohort study used data collected from April 1, 2003, to February 26, 2016, from mother-child pairs from the Human Early-Life Exposome project, a collaborative network of 6 ongoing, population-based prospective birth cohort studies from 6 European countries (France, Greece, Lithuania, Norway, Spain, and the UK). Data were analyzed from April 1, 2021, to January 31, 2022.
Exposures: Three organochlorine pesticides, 5 polychlorinated biphenyls, 2 polybrominated diphenyl ethers (PBDEs), 3 phenols, 4 parabens, 10 phthalates, 4 organophosphate pesticides, 5 perfluoroalkyl substances, and 9 metals.
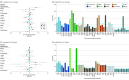
Main outcomes and measures: Child serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyltransferase (GGT), and CK-18 were measured at 6 to 11 years of age. Risk for liver injury was defined as having ALT, AST, and/or GGT levels above the 90th percentile. Associations of liver injury or cytokeratin 18 (CK-18) levels with each chemical group among the 45 EDCs measured in maternal blood or urine samples collected in pregnancy were estimated using 2 complimentary exposure-mixture methods: bayesian weighted quantile sum (BWQS) and bayesian kernel machine regression.
Results: The study included 1108 mothers (mean [SD] age at birth, 31.0 [4.7] years) and their singleton children (mean [SD] age at liver assessment, 8.2 [1.6] years; 598 [54.0%] boys). Results of the BWQS method indicated increased odds of liver injury per exposure-mixture quartile increase for organochlorine pesticides (odds ratio [OR], 1.44 [95% credible interval (CrI), 1.21-1.71]), PBDEs (OR, 1.57 [95% CrI, 1.34-1.84]), perfluoroalkyl substances (OR, 1.73 [95% CrI, 1.45-2.09]), and metals (OR, 2.21 [95% CrI, 1.65-3.02]). Decreased odds of liver injury were associated with high-molecular-weight phthalates (OR, 0.74 [95% CrI, 0.60-0.91]) and phenols (OR, 0.66 [95% CrI, 0.54-0.78]). Higher CK-18 levels were associated with a 1-quartile increase in polychlorinated biphenyls (β, 5.84 [95% CrI, 1.69-10.08] IU/L) and PBDEs (β, 6.46 [95% CrI, 3.09-9.92] IU/L). Bayesian kernel machine regression showed associations in a similar direction as BWQS for all EDCs and a nonlinear association between phenols and CK-18 levels.
Conclusions and relevance: With a combination of 2 state-of-the-art exposure-mixture approaches, consistent evidence suggests that prenatal exposures to EDCs are associated with higher risk for liver injury and CK-18 levels and constitute a potential risk factor for pediatric nonalcoholic fatty liver disease.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

