An Enzymatically Cleavable Tripeptide Linker for Maximizing the Therapeutic Index of Antibody-Drug Conjugates
- PMID: 35793453
- PMCID: PMC9452487
- DOI: 10.1158/1535-7163.MCT-22-0362
An Enzymatically Cleavable Tripeptide Linker for Maximizing the Therapeutic Index of Antibody-Drug Conjugates
Abstract
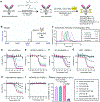
Valine-citrulline is a protease-cleavable linker commonly used in many drug delivery systems, including antibody-drug conjugates (ADC) for cancer therapy. However, its suboptimal in vivo stability can cause various adverse effects such as neutropenia and hepatotoxicity, leading to dose delays or treatment discontinuation. Here, we report that glutamic acid-glycine-citrulline (EGCit) linkers have the potential to solve this clinical issue without compromising the ability of traceless drug release and ADC therapeutic efficacy. We demonstrate that our EGCit ADC resists neutrophil protease-mediated degradation and spares differentiating human neutrophils. Notably, our anti-HER2 ADC shows almost no sign of blood and liver toxicity in healthy mice at 80 mg kg-1. In contrast, at the same dose level, the FDA-approved anti-HER2 ADCs Kadcyla and Enhertu show increased levels of serum alanine aminotransferase and aspartate aminotransferase and morphologic changes in liver tissues. Our EGCit conjugates also exert greater antitumor efficacy in multiple xenograft tumor models compared with Kadcyla and Enhertu. This linker technology could substantially broaden the therapeutic windows of ADCs and other drug delivery agents, providing clinical options with improved efficacy and safety.
©2022 American Association for Cancer Research.
Conflict of interest statement
Conflicts of Interest Statement
Y.A., C.M.Y., N.Z., Z.A., and K.T. are named inventors on patent applications relating to the work (PCT/US2018/034363, US-2020-0115326-A1, EU18804968.8-1109/3630189). S.Y.Y.H., Y.A., C.M.Y., N.Z., Z.A., and K.T. are named inventors on a pending patent application relating to the work. All patent applications were filed by the Board of Regents of the University of Texas System. The remaining authors declare no competing interests.
Figures





References
-
- Khongorzul P, Ling CJ, Khan FU, Ihsan AU, Zhang J. Antibody–Drug Conjugates: A Comprehensive Review. Mol Cancer Res. American Association for Cancer Research; 2020;18:3–19. - PubMed
-
- Lehar SM, Pillow T, Xu M, Staben L, Kajihara KK, Vandlen R, et al. Novel antibody–antibiotic conjugate eliminates intracellular S. aureus. Nature. Nature Publishing Group; 2015;527:323–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

