Anti-Extra Domain B Splice Variant of Fibronectin Antibody-Drug Conjugate Eliminates Tumors with Enhanced Efficacy When Combined with Checkpoint Blockade
- PMID: 35793468
- PMCID: PMC9446899
- DOI: 10.1158/1535-7163.MCT-22-0099
Anti-Extra Domain B Splice Variant of Fibronectin Antibody-Drug Conjugate Eliminates Tumors with Enhanced Efficacy When Combined with Checkpoint Blockade
Abstract
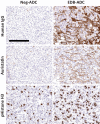
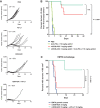
Extra domain B splice variant of fibronectin (EDB+FN) is an extracellular matrix protein (ECM) deposited by tumor-associated fibroblasts, and is associated with tumor growth, angiogenesis, and invasion. We hypothesized that EDB+FN is a safe and abundant target for therapeutic intervention with an antibody-drug conjugate (ADC). We describe the generation, pharmacology, mechanism of action, and safety profile of an ADC specific for EDB+FN (EDB-ADC). EDB+FN is broadly expressed in the stroma of pancreatic, non-small cell lung (NSCLC), breast, ovarian, head and neck cancers, whereas restricted in normal tissues. In patient-derived xenograft (PDX), cell-line xenograft (CLX), and mouse syngeneic tumor models, EDB-ADC, conjugated to auristatin Aur0101 through site-specific technology, demonstrated potent antitumor growth inhibition. Increased phospho-histone H3, a pharmacodynamic biomarker of response, was observed in tumor cells distal to the target site of tumor ECM after EDB-ADC treatment. EDB-ADC potentiated infiltration of immune cells, including CD3+ T lymphocytes into the tumor, providing rationale for the combination of EDB-ADC with immune checkpoint therapy. EDB-ADC and anti-PD-L1 combination in a syngeneic breast tumor model led to enhanced antitumor activity with sustained tumor regressions. In nonclinical safety studies in nonhuman primates, EDB-ADC had a well-tolerated safety profile without signs of either on-target toxicity or the off-target effects typically observed with ADCs that are conjugated through conventional conjugation methods. These data highlight the potential for EDB-ADC to specifically target the tumor microenvironment, provide robust therapeutic benefits against multiple tumor types, and enhance activity antitumor in combination with checkpoint blockade.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures



Comment in
- 1535-7163. doi: 10.1158/1535-7163.MCT-21-9-HI
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

