Switches between biologics in patients with moderate-to-severe psoriasis: results from the French cohort PSOBIOTEQ
- PMID: 35793473
- PMCID: PMC9796114
- DOI: 10.1111/jdv.18409
Switches between biologics in patients with moderate-to-severe psoriasis: results from the French cohort PSOBIOTEQ
Abstract
Background: Biologics are the cornerstone of treatment of patients with moderate-to-severe plaque psoriasis and switches between biologics are frequently needed to maintain clinical improvement over time.
Objectives: The main purpose of this study was to describe precisely switches between biologics and how their pattern changed over time with the recent availability of new biologic agents.
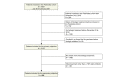
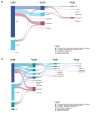
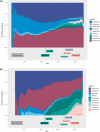
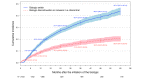
Methods: We included patients receiving a first biologic agent in the Psobioteq multicenter cohort of adults with moderate-to-severe psoriasis receiving systemic treatment. We described switches between biologics with chronograms, Sankey and Sunburst diagrams, assessed cumulative incidence of first switch by competing risks survival analysis and reasons for switching. We assessed the factors associated with the type of switch (intra-class - i.e. within the same therapeutic class - vs. inter-class) in patients switching from a TNF-alpha inhibitor using multivariate logistic regression.
Results: A total of 2153 patients was included. The cumulative incidence of switches from first biologic was 34% at 3 years. Adalimumab and ustekinumab were the most prescribed biologic agents as first and second lines of treatment. The main reason for switching was loss of efficacy (72%), followed by adverse events (11%). Patients receiving a TNF-alpha inhibitor before 2016 mostly switched to ustekinumab, whereas those switching in 2016 or after mostly switched to an IL-17 inhibitor. Patients switching from a first-line TNF-alpha inhibitor before 2016 were more likely to switch to another TNF-alpha inhibitor compared with patients switching since 2018. Patients switching from etanercept were more likely to receive another TNF-alpha inhibitor rather than another therapeutic class of bDMARD compared with patients switching from adalimumab.
Conclusion: This study described the switching patterns of biologic treatments and showed how they changed over time, due to the availability of the new biologic agents primarily IL-17 inhibitors.
© 2022 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Figures


References
-
- Richard MA, Corgibet F, Beylot‐Barry M et al. Sex‐ and age‐adjusted prevalence estimates of five chronic inflammatory skin diseases in France: results of the « OBJECTIFS PEAU » study. J Eur Acad Dermatol Venereol JEADV 2018; 32: 1967–1971. - PubMed
-
- Amatore F, Villani A‐P, Tauber M, Viguier M, Guillot B. Psoriasis research Group of the French Society of dermatology (Groupe de Recherche Sur le psoriasis de la Société Française de Dermatologie). French guidelines on the use of systemic treatments for moderate‐to‐severe psoriasis in adults. J Eur Acad Dermatol Venereol JEADV. 2019; 33: 464–483. - PMC - PubMed
-
- Özkur E, Kıvanç Altunay İ, Oğuz Topal İ et al. Switching biologics in the treatment of psoriasis: a multicenter experience. Dermatol Basel Switz 2021; 237: 22–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

