Sex differences in autonomic responses to stress: implications for cardiometabolic physiology
- PMID: 35793480
- PMCID: PMC9448273
- DOI: 10.1152/ajpendo.00058.2022
Sex differences in autonomic responses to stress: implications for cardiometabolic physiology
Abstract
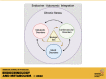
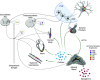
Chronic stress is a significant risk factor for negative health outcomes. Furthermore, imbalance of autonomic nervous system control leads to dysregulation of physiological responses to stress and contributes to the pathogenesis of cardiometabolic and psychiatric disorders. However, research on autonomic stress responses has historically focused on males, despite evidence that females are disproportionality affected by stress-related disorders. Accordingly, this mini-review focuses on the influence of biological sex on autonomic responses to stress in humans and rodent models. The reviewed literature points to sex differences in the consequences of chronic stress, including cardiovascular and metabolic disease. We also explore basic rodent studies of sex-specific autonomic responses to stress with a focus on sex hormones and hypothalamic-pituitary-adrenal axis regulation of cardiovascular and metabolic physiology. Ultimately, emerging evidence of sex differences in autonomic-endocrine integration highlights the importance of sex-specific studies to understand and treat cardiometabolic dysfunction.
Keywords: androgen; autonomic balance; chronic stress; estrogen; glucocorticoid.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
References
-
- Anand SS, Yi Q, Gerstein H, Lonn E, Jacobs R, Vuksan V, Teo K, Davis B, Montague P, Yusuf S; Study of Health Assessment and Risk Evaluation in Aboriginal Peoples Investigators. Relationship of metabolic syndrome and fibrinolytic dysfunction to cardiovascular disease. Circulation 108: 420–425, 2003. doi:10.1161/01.CIR.0000080884.27358.49. - DOI - PubMed
-
- McIntyre RS, Rasgon NL, Kemp DE, Nguyen HT, Law CWY, Taylor VH, Woldeyohannes HO, Alsuwaidan MT, Soczynska JK, Kim B, Lourenco MT, Kahn LS, Goldstein BI. Metabolic syndrome and major depressive disorder: co-occurrence and pathophysiologic overlap. Curr Diab Rep 9: 51–59, 2009. doi:10.1007/s11892-009-0010-0. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

