Effects of auditory sleep modulation approaches on brain oscillatory and cardiovascular dynamics
- PMID: 35793672
- PMCID: PMC9453626
- DOI: 10.1093/sleep/zsac155
Effects of auditory sleep modulation approaches on brain oscillatory and cardiovascular dynamics
Abstract
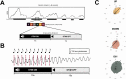
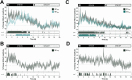
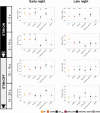
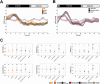
Slow waves, the hallmark feature of deep nonrapid eye movement sleep, do potentially drive restorative effects of sleep on brain and body functions. Sleep modulation techniques to elucidate the functional role of slow waves thus have gained large interest. Auditory slow wave stimulation is a promising tool; however, directly comparing auditory stimulation approaches within a night and analyzing induced dynamic brain and cardiovascular effects are yet missing. Here, we tested various auditory stimulation approaches in a windowed, 10 s ON (stimulations) followed by 10 s OFF (no stimulations), within-night stimulation design and compared them to a SHAM control condition. We report the results of three studies and a total of 51 included nights and found a large and global increase in slow-wave activity (SWA) in the stimulation window compared to SHAM. Furthermore, slow-wave dynamics were most pronouncedly increased at the start of the stimulation and declined across the stimulation window. Beyond the changes in brain oscillations, we observed, for some conditions, a significant increase in the mean interval between two heartbeats within a stimulation window, indicating a slowing of the heart rate, and increased heart rate variability derived parasympathetic activity. Those cardiovascular changes were positively correlated with the change in SWA, and thus, our findings provide insight into the potential of auditory slow wave enhancement to modulate cardiovascular restorative conditions during sleep. However, future studies need to investigate whether the potentially increased restorative capacity through slow-wave enhancements translates into a more rested cardiovascular system on a subsequent day.
Keywords: auditory stimulation; cardiovascular recovery; heart rate variability; slow waves.
© Sleep Research Society 2022. Published by Oxford University Press on behalf of the Sleep Research Society.
Figures


Comment in
-
Acoustic enhancement of slow wave sleep-timing and tone: "the details create the big picture".Sleep. 2022 Oct 10;45(10):zsac191. doi: 10.1093/sleep/zsac191. Sleep. 2022. PMID: 35985359 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

