Inhibition of stearoyl-CoA desaturase 1 (SCD1) enhances the antitumor T cell response through regulating β-catenin signaling in cancer cells and ER stress in T cells and synergizes with anti-PD-1 antibody
- PMID: 35793868
- PMCID: PMC9260842
- DOI: 10.1136/jitc-2022-004616
Inhibition of stearoyl-CoA desaturase 1 (SCD1) enhances the antitumor T cell response through regulating β-catenin signaling in cancer cells and ER stress in T cells and synergizes with anti-PD-1 antibody
Abstract
Background: Understanding the mechanisms of non-T cell inflamed tumor microenvironment (TME) and their modulation are important to improve cancer immunotherapies such as immune checkpoint inhibitors. The involvement of various immunometabolisms has recently been indicated in the formation of immunosuppressive TME. In this study, we investigated the immunological roles of stearoyl-CoA desaturase 1 (SCD1), which is essential for fatty acid metabolism, in the cancer immune response.
Methods: We investigated the roles of SCD1 by inhibition with the chemical inhibitor or genetic manipulation in antitumor T cell responses and the therapeutic effect of anti-programmed cell death protein 1 (anti-PD-1) antibody using various mouse tumor models, and their cellular and molecular mechanisms. The roles of SCD1 in human cancers were also investigated by gene expression analyses of colon cancer tissues and by evaluating the related free fatty acids in sera obtained from patients with non-small cell lung cancer who were treated with anti-PD-1 antibody.
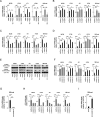
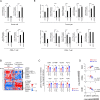
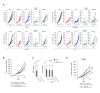
Results: Systemic administration of a SCD1 inhibitor in mouse tumor models enhanced production of CCL4 by cancer cells through reduction of Wnt/β-catenin signaling and by CD8+ effector T cells through reduction of endoplasmic reticulum stress. It in turn promoted recruitment of dendritic cells (DCs) into the tumors and enhanced the subsequent induction and tumor accumulation of antitumor CD8+ T cells. SCD1 inhibitor was also found to directly stimulate DCs and CD8+ T cells. Administration of SCD1 inhibitor or SCD1 knockout in mice synergized with an anti-PD-1 antibody for its antitumor effects in mouse tumor models. High SCD1 expression was observed in one of the non-T cell-inflamed subtypes in human colon cancer, and serum SCD1 related fatty acids were correlated with response rates and prognosis of patients with non-small lung cancer following anti-PD-1 antibody treatment.
Conclusions: SCD1 expressed in cancer cells and immune cells causes immunoresistant conditions, and its inhibition augments antitumor T cells and therapeutic effects of anti-PD-1 antibody. Therefore, SCD1 is an attractive target for the development of new diagnostic and therapeutic strategies to improve current cancer immunotherapies including immune checkpoint inhibitors.
Keywords: CD8-Positive T-Lymphocytes; Drug Therapy, Combination; Immunotherapy; Tumor Microenvironment.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
