Efficacy of anti-PD-1 and ipilimumab alone or in combination in acral melanoma
- PMID: 35793872
- PMCID: PMC9260790
- DOI: 10.1136/jitc-2022-004668
Efficacy of anti-PD-1 and ipilimumab alone or in combination in acral melanoma
Abstract
Background: Acral melanoma is a rare melanoma subtype with poor prognosis. Importantly, these patients were not identified as a specific subgroup in the landmark melanoma trials involving ipilimumab and the anti-programmed cell death protein-1 (PD-1) agents nivolumab and pembrolizumab. There is therefore an absence of prospective clinical trial evidence regarding the efficacy of checkpoint inhibitors (CPIs) in this population. Acral melanoma has lower tumor mutation burden (TMB) than other cutaneous sites, and primary site is associated with differences in TMB. However the impact of this on the effectiveness of immune CPIs is unknown. We examined the efficacy of CPIs in acral melanoma, including by primary site.
Methods: Patients with unresectable stage III/IV acral melanoma treated with CPI (anti-PD-1 and/or ipilimumab) were studied. Multivariable logistic and Cox regression analyses were conducted. Primary outcome was objective response rate (ORR); secondary outcomes were progression-free survival (PFS) and overall survival (OS).
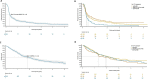
Results: In total, 325 patients were included: 234 (72%) plantar, 69 (21%) subungual and 22 (7%) palmar primary sites. First CPI included: 184 (57%) anti-PD-1, 59 (18%) anti-PD-1/ipilimumab combination and 82 (25%) ipilimumab. ORR was significantly higher with initial anti-PD-1/ipilimumab compared with anti-PD-1 (43% vs 26%, HR 2.14, p=0.0004) and significantly lower with ipilimumab (15% vs 26%, HR 0.49, p=0.0016). Landmark PFS at 1 year was highest for anti-PD-1/ipilimumab at 34% (95% CI 24% to 49%), compared with 26% (95% CI 20% to 33%) with anti-PD-1 and 10% (95% CI 5% to 19%) with ipilimumab. Despite a trend for increased PFS, anti-PD-1/ipilimumab combination did not significantly improve PFS (HR 0.85, p=0.35) or OS over anti-PD-1 (HR 1.30, p=0.16), potentially due to subsequent therapies and high rates of acquired resistance. No outcome differences were found between primary sites.
Conclusion: While the ORR to anti-PD-1/ipilimumab was significantly higher than anti-PD-1 and PFS numerically higher, in this retrospective cohort this benefit did not translate to improved OS. Future trials should specifically include patients with acral melanoma, to help determine the optimal management of this important melanoma subtype.
Keywords: CTLA-4 Antigen; Immunotherapy; Melanoma; Programmed Cell Death 1 Receptor.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: PB: sponsorship: Bristol Myers Squibb, MSD, Novartis; paid speaker: Novartis. AS: advisory board: Bristol Myers Squibb, Immunocore, Novartis, Castle Biosciences; trial support to institution: Bristol Myers Squibb, Imunocore, Xcovery, Polaris, Novartis, Pfizer, Checkmate Pharmaceuticals, Foghorn Therapeautics. AZ: travel support: Novartis, Sanofi Grenzyme, Sun Pharma, Bristol Myers Squibb. JM: consultant advisor: Merck/Pfizer, Merck Sharp & Dohme, Amgen, Novartis, Sanofi, Bristol Myers Squibb and Pierre Fabre; travel support: Ultrasun, L’Oreal, Merck Sharp & Dohme, Bristol Myers Squibb, Pierre Fabre. MW: grants, personal fees, non-financial support and other: Novartis, BMS, Merck/MSD; personal fees, and other: Merck Serono, Amgen, Sanofi/Regeneron, Pierre Fabre, Roche, Takeda, Medac. LS: honoraria: MSD, Roche, Novartis, Shanghai Junshi Biosciences, Oriengene. TL: consultant: MSD, Novartis, Pierre Fabre. CR: consultant for Novartis, BMS, Merck, MSD, Sanofi, Pierre Fabre, AstraZeneca, Roche, and scientic founder of Aglaia Therapeutics CA: Travel accommodations-Meetings: Roche, Bristol Myers Squibb, Amgen. JKS: sponsorship: MSD, Amgen; honoraria: Novartis. MJM: consultant advisor: AstraZeneca, Nektar Therapeutics, Catalyst Pharmaceuticals Immunia, Istari Oncology; paid speaker: Bristol Myers Squibb. RR-T: advisory board fees: Novartis, Bristol Myer Squibb, Merck, Sharp and Dohme, Pfizer; honoraria: Bristol Myer Squibb, Roche, AstraZeneca, Merck Sharp and Dohme, Novartis. AM: honoraria from Novartis. LZ: consultant and/or honoraria: Bristol Myers Squibb, Merck Sharp & Dohme, Novartis, Pierre Fabre, Sanofi, Sunpharma; travel support: Amgen, Merck Sharp & Dohme, BristolMyers Squibb, Pierre Fabre, Sanofi, Sunpharma, Novartis. CB (all paid to the institute except TRV): advisory board: Bristol Myers Squibb, Merck Sharp & Dohme, Roche, Novartis, GlaxoSmithKline, AstraZeneca, Pfizer, Lilly, GenMab, Pierre Fabre, Third Rock Ventures; research funding: Bristol Myers Squibb, Novartis, NanoString; stockownership & co-founder: Immagene BV. ER: consultant advisor or paid speaker: Sanofi, Amgen, BMS. KK: consultant or/and honoraria: Amgen, Roche, Bristol Myers Squibb, Merck Sharp and Dohme, Pierre Fabre, Novartis; travel support: Amgen, Merck Sharp and Dohme, Bristol Myers Squibb, Amgen, Pierre Fabre, Medac, Novartis. PAA: consultant/advisory role: Bristol Myers Squibb, Roche-Genentech, Merck Sharp & Dohme, Novartis, Merck Serono, Pierre-Fabre, AstraZeneca, Sun Pharma, Sanofi, Idera, Sandoz, Immunocore, 4SC, Italfarmaco, Nektar, Boehringer-Ingelheim, Eisai, Regeneron, Daiichi Sankyo, Pfizer, Oncosec, Nouscom, Lunaphore, Seagen, iTeos; research funding: Bristol Myers Squibb, Roche-Genentech, Pfizer, Sanofi. OM: consulting/advisory roles: Bristol Myers Squibb, MSD, Roche, Novartis, Amgen, Pierre Fabre, Neracare; research grants: Bristol Myers Squibb, MSD, Amgen. PCL: Consultant advisor or paid speaker: Bristol Myers Squibb, MSD, Novartis, Pierre Fabre, Amgen, Nektar. Research funding: Bristol Myers Squibb, Pierre Fabre. DBJ: advisory boards/consultant: BMS, Catalyst Biopharma, Iovance, Jansen, Mallinckrodt, Merck, Mosaic ImmunoEngineering, Novartis, Oncosec, Pfizer, and Targovax. Research funding: BMS and Incyte. RP: advisory boards: Pierre Faber, Bayer, Novartis, Biosceptre, Bristol Myers Squibb, Cybrexa, Ellipses, CV6 Therapeutics, Astex Therapeutics, Medivir, GammaDelta Therapeutics, Sanofi Aventis; paid speaker: AstraZeneca, Novartis, Bayer, Tesaro, Bristol Myers Squibb. CL: BMS: Research grant, Honoraria, Consultancy, Speakers bureau, Travel accommodations, Meetings, Advisory role, advisory board. MSD: Honoraria, Consultancy, Advisory role, advisory board, Travel accommodations-Meetings. Novartis: Honoraria, Consultancy, Speakers bureau, Advisory role, advisory board. Amgen: Honoraria, Consultancy, Speakers bureau, advisory board. Roche: Research grant, Honoraria, Consultancy, Speakers bureau, Advisory role, advisory board. Avantis Medical Systems: Board. Pierre-Fabre/Pfizer/Incyte: Honoraria. BN: Personal financial compensation from Roche, Bristol Myers Squibb, Merck Sharp & Dohme, Novartis, Pfizer, Amgen and AstraZeneca for public speaking, consultancy and participation in advisory board meetings. My institution (UZ Brussel) received funding related to research projects conducted by my academic research team from Pfizer, Novartis, Roche, and Merck-Serono. RJS: Consultant: AstraZeneca, Bristol Myers Squibb, Eisai, Iovance, Merck, Novartis, Pfizer; research funding: Merck. OH: consultant advisor: Aduro, Akeso, Amgen, Beigene, Bioatla, BMS, Roche Genentech, GSK, Immunocore, Idera, Incyte, Janssen, Merck, Nextcure, Novartis, Pfizer, Sanofi/Regeneron, Seattle Genetics, Tempus, Zelluna; speaker bureau: BMS, Novartis, Pfizer, Sanofi/Regeneron; contracted research (for institution): Arcus, Aduro, Akeso, Amgen, Bioatla, BMS, CytomX, Exelixis, Roche Genentech, GSK, Immunocore, Idera, Incyte, Iovance, Merck, Moderna, Merck-Serono, NextCure, Novartis, Pfizer, Sanofi/Regeneron, Seattle Genetics, Torque, Zelluna. GAM: reimbursement of trials costs to the Peter MacCallum Cancer Centre: Array/Pfizer Roche/Genetech; non-reimbursed advisor: Novartis, Bristol Myers Squibb. AMH: advisory board member for Amgen, Bristol Myers Squibb, Merck Sharp and Dohme, Novartis, Pierre-Fabre, and Qbiotics, GVL: advisor: Aduro Biotech, Agenus, Amgen, Array Biopharma, Boehringer, Bristol Myers Squibb, Evaxion, Highlight Therapeutics S.L, Merck Sharp and Dohme, Novartis, Pierre Fabre, QBiotics, Regeneron, Specialised Therapeutics Australia. AM: advisory board: Bristol Myers Squibb, Merck Sharp and Dohme, Novartis, Roche, Pierre-Fabre, QBiotics. MC: consultant advisor: Amgen, Bristol Myers Squibb, Eisai, Ideaya, Merck Sharp and Dohme, Nektar, Novartis, Oncosec, Pierre-Fabre, Qbiotics, Regeneron, Roche; honoraria: Bristol Myers Squibb, Merck Sharp and Dohme, Novartis. All other authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
