Berberine treats atherosclerosis via a vitamine-like effect down-regulating Choline-TMA-TMAO production pathway in gut microbiota
- PMID: 35794102
- PMCID: PMC9259588
- DOI: 10.1038/s41392-022-01027-6
Berberine treats atherosclerosis via a vitamine-like effect down-regulating Choline-TMA-TMAO production pathway in gut microbiota
Abstract
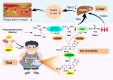
Trimethylamine-N-oxide (TMAO) derived from the gut microbiota is an atherogenic metabolite. This study investigates whether or not berberine (BBR) could reduce TMAO production in the gut microbiota and treat atherosclerosis. Effects of BBR on TMAO production in the gut microbiota, as well as on plaque development in atherosclerosis were investigated in the culture of animal intestinal bacterial, HFD-fed animals and atherosclerotic patients, respectively. We found that oral BBR in animals lowers TMAO biosynthesis in intestine through interacting with the enzyme/co-enzyme of choline-trimethylamine lyase (CutC) and flavin-containing monooxygenase (FMO) in the gut microbiota. This action was performed by BBR's metabolite dihydroberberine (a reductive BBR by nitroreductase in the gut microbiota), via a vitamine-like effect down-regulating Choline-TMA-TMAO production pathway. Oral BBR decreased TMAO production in animal intestine, lowered blood TMAO and interrupted plaque formation in blood vessels in the HFD-fed hamsters. Moreover, 21 patients with atherosclerosis exhibited the average decrease of plaque score by 3.2% after oral BBR (0.5 g, bid) for 4 months (*P < 0.05, n = 21); whereas the plaque score in patients treated with rosuvastatin plus aspirin, or clopidogrel sulfate or ticagrelor (4 months, n = 12) increased by 1.9%. TMA and TMAO in patients decreased by 38 and 29% in faeces (*P < 0.05; *P < 0.05), and 37 and 35% in plasma (***P < 0.001; *P < 0.05), after 4 months on BBR. BBR might treat atherosclerotic plaque at least partially through decreasing TMAO in a mode of action similar to that of vitamins.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

