Periarteriolar spaces modulate cerebrospinal fluid transport into brain and demonstrate altered morphology in aging and Alzheimer's disease
- PMID: 35794106
- PMCID: PMC9259669
- DOI: 10.1038/s41467-022-31257-9
Periarteriolar spaces modulate cerebrospinal fluid transport into brain and demonstrate altered morphology in aging and Alzheimer's disease
Abstract
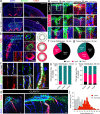
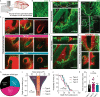
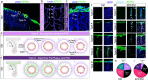
Perivascular spaces (PVS) drain brain waste metabolites, but their specific flow paths are debated. Meningeal pia mater reportedly forms the outermost boundary that confines flow around blood vessels. Yet, we show that pia is perforated and permissive to PVS fluid flow. Furthermore, we demonstrate that pia is comprised of vascular and cerebral layers that coalesce in variable patterns along leptomeningeal arteries, often merging around penetrating arterioles. Heterogeneous pial architectures form variable sieve-like structures that differentially influence cerebrospinal fluid (CSF) transport along PVS. The degree of pial coverage correlates with macrophage density and phagocytosis of CSF tracer. In vivo imaging confirms transpial influx of CSF tracer, suggesting a role of pia in CSF filtration, but not flow restriction. Additionally, pial layers atrophy with age. Old mice also exhibit areas of pial denudation that are not observed in young animals, but pia is unexpectedly hypertrophied in a mouse model of Alzheimer's disease. Moreover, pial thickness correlates with improved CSF flow and reduced β-amyloid deposits in PVS of old mice. We show that PVS morphology in mice is variable and that the structure and function of pia suggests a previously unrecognized role in regulating CSF transport and amyloid clearance in aging and disease.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA. Evidence for a 'paravascular' fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985;326:47–63. doi: 10.1016/0006-8993(85)91383-6. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

