Decatungstate-catalyzed radical disulfuration through direct C-H functionalization for the preparation of unsymmetrical disulfides
- PMID: 35794128
- PMCID: PMC9259577
- DOI: 10.1038/s41467-022-31617-5
Decatungstate-catalyzed radical disulfuration through direct C-H functionalization for the preparation of unsymmetrical disulfides
Abstract
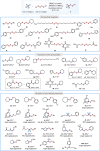
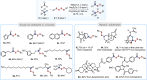
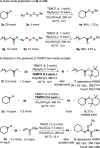
Unsymmetrical disulfides are widely found in the areas of food chemistry, pharmaceutical industry, chemical biology and polymer science. Due the importance of such disulfides in various fields, general methods for the nondirected intermolecular disulfuration of C-H bonds are highly desirable. In this work, the conversion of aliphatic C(sp3)-H bonds and aldehydic C(sp2)-H bonds into the corresponding C-SS bonds with tetrasulfides (RSSSSR) as radical disulfuration reagents is reported. The decatungstate anion ([W10O32]4-) as photocatalyst is used for C-radical generation via intermolecular hydrogen atom transfer in combination with cheap sodium persulfate (Na2S2O8) as oxidant. Herein a series of valuable acyl alkyl disulfides, important precursors for the generation of RSS-anions, and unsymmetrical dialkyl disulfides are synthesized using this direct approach. To demonstrate the potential of the method for late-stage functionalization, approved drugs and natural products were successfully C-H functionalized.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
The merger of decatungstate and copper catalysis to enable aliphatic C(sp3)-H trifluoromethylation.Nat Chem. 2020 May;12(5):459-467. doi: 10.1038/s41557-020-0436-1. Epub 2020 Mar 16. Nat Chem. 2020. PMID: 32203440
-
Origin and Regioselectivity of Direct Hydrogen Atom Transfer Mechanism of C(sp3)-H Arylation by [W10O32]4-/Ni Metallaphotoredox Catalysis.Inorg Chem. 2021 Dec 20;60(24):18706-18714. doi: 10.1021/acs.inorgchem.1c02118. Epub 2021 Nov 26. Inorg Chem. 2021. PMID: 34823352
-
Theoretical insight into decatungstate photocatalyzed alkylation of N-tosylimine via hydrogen atom transfer and proton-coupled electron transfer.Dalton Trans. 2022 May 24;51(20):7928-7935. doi: 10.1039/d2dt00927g. Dalton Trans. 2022. PMID: 35543136
-
Direct C-H Sulfuration: Synthesis of Disulfides, Dithiocarbamates, Xanthates, Thiocarbamates and Thiocarbonates.Chem Asian J. 2024 May 2;19(9):e202400124. doi: 10.1002/asia.202400124. Epub 2024 Apr 11. Chem Asian J. 2024. PMID: 38421239 Review.
-
Electronic control over site-selectivity in hydrogen atom transfer (HAT) based C(sp3)-H functionalization promoted by electrophilic reagents.Chem Soc Rev. 2022 Mar 21;51(6):2171-2223. doi: 10.1039/d1cs00556a. Chem Soc Rev. 2022. PMID: 35229835 Review.
Cited by
-
Chemodivergent alkylation of trifluoromethyl alkenes via photocatalytic coupling with alkanes.Green Chem. 2024 Oct 4;26(22):11196-11205. doi: 10.1039/d4gc04176c. eCollection 2024 Nov 11. Green Chem. 2024. PMID: 39398964 Free PMC article.
-
Engineered Half-Unit-Cell MoS2/ZnIn2S4 Monolayer Photocatalysts and Adsorbed Hydroxyl Radicals-Assisted Activation of Cα-H Bond for Efficient Cβ-O Bond Cleavage in Lignin to Aromatic Monomers.ACS Appl Mater Interfaces. 2024 Sep 11;16(36):47724-47740. doi: 10.1021/acsami.4c10515. Epub 2024 Aug 30. ACS Appl Mater Interfaces. 2024. PMID: 39215384 Free PMC article.
-
Radical approaches to C-S bonds.Nat Rev Chem. 2023 Aug;7(8):573-589. doi: 10.1038/s41570-023-00505-x. Epub 2023 Jun 21. Nat Rev Chem. 2023. PMID: 37344618 Review.
-
Tetrabutylammonium decatungstate-catalyzed hydroalkylation/alkoxylation of 3-methyleneisoindolin-1-ones with alcohols/ethers through hydrogen atom transfer process.RSC Adv. 2025 Aug 26;15(37):30285-30289. doi: 10.1039/d5ra05071e. eCollection 2025 Aug 22. RSC Adv. 2025. PMID: 40874149 Free PMC article.
-
SO2F2 mediated click chemistry enables modular disulfide formation in diverse reaction media.Nat Commun. 2024 Sep 27;15(1):8325. doi: 10.1038/s41467-024-52606-w. Nat Commun. 2024. PMID: 39333088 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

