Decatungstate-catalyzed radical disulfuration through direct C-H functionalization for the preparation of unsymmetrical disulfides
- PMID: 35794128
- PMCID: PMC9259577
- DOI: 10.1038/s41467-022-31617-5
Decatungstate-catalyzed radical disulfuration through direct C-H functionalization for the preparation of unsymmetrical disulfides
Abstract
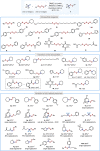
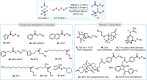
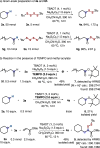
Unsymmetrical disulfides are widely found in the areas of food chemistry, pharmaceutical industry, chemical biology and polymer science. Due the importance of such disulfides in various fields, general methods for the nondirected intermolecular disulfuration of C-H bonds are highly desirable. In this work, the conversion of aliphatic C(sp3)-H bonds and aldehydic C(sp2)-H bonds into the corresponding C-SS bonds with tetrasulfides (RSSSSR) as radical disulfuration reagents is reported. The decatungstate anion ([W10O32]4-) as photocatalyst is used for C-radical generation via intermolecular hydrogen atom transfer in combination with cheap sodium persulfate (Na2S2O8) as oxidant. Herein a series of valuable acyl alkyl disulfides, important precursors for the generation of RSS-anions, and unsymmetrical dialkyl disulfides are synthesized using this direct approach. To demonstrate the potential of the method for late-stage functionalization, approved drugs and natural products were successfully C-H functionalized.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

