REL-1017 (esmethadone; D-methadone) does not cause reinforcing effect, physical dependence and withdrawal signs in Sprague Dawley rats
- PMID: 35794162
- PMCID: PMC9259683
- DOI: 10.1038/s41598-022-15055-3
REL-1017 (esmethadone; D-methadone) does not cause reinforcing effect, physical dependence and withdrawal signs in Sprague Dawley rats
Abstract
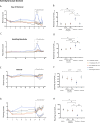
REL-1017 (esmethadone, D-methadone) is the opioid-inactive d-isomer of racemic D,L-methadone. REL-1017 may exert antidepressant effects via uncompetitive N-methyl-D-aspartate receptor (NMDAR) channel block. As REL-1017 is expected to exert central nervous system activity, full characterization of its abuse potential is warranted. We evaluated lack of reinforcing effect, physical dependence, and withdrawal of REL-1017 in Sprague Dawley rats. (1) Self-administration Study Rats were trained to self-administer oxycodone intravenously (IV) and then were subjected to 3-day substitution tests where saline, oxycodone, and REL-1017 were self-delivered IV by a fixed number of lever presses; (2) Drug Discontinuation Study Rats were treated for 30 days by oral gavage with vehicle, REL-1017, ketamine or morphine and evaluated for withdrawal with functional observational batteries (FOBs). In the self-administration study, rats treated with saline, vehicle, and all REL-1017 doses showed the typical "extinction burst" pattern of response, characterized by an initial rapid increase of lever-pressing followed by a rapid decrease over 3 days. Rats treated with oxycodone maintained stable self-injection, as expected for reinforcing stimuli. In the withdrawal study, REL-1017 did not engender either morphine or ketamine withdrawal signs over 9 days following abrupt discontinuation of drug exposure. REL-1017 showed no evidence of abuse potential and did not engender withdrawal symptomatology.
© 2022. The Author(s).
Conflict of interest statement
This research was performed at Charles River Laboratories and was sponsored by Relmada Therapeutics. Dr Gauvin is an employee at Charles River Laboratories. Drs. Manfredi, Inturrisi, Pappagallo, Shram, Henningfield, Buchhalter, Ashworth, Lanier, Folli are paid consultants of Relmada Therapeutics. Sergio Traversa is an employee at Relmada Therapeutics. Drs Inturrisi and Manfredi are inventors on esmethadone patents and other patents and patent applications. Francesco Bifari has no competing interests.
Figures





Similar articles
-
The novel uncompetitive NMDA receptor antagonist esmethadone (REL-1017) has no meaningful abuse potential in recreational drug users.Transl Psychiatry. 2023 Jun 7;13(1):192. doi: 10.1038/s41398-023-02473-8. Transl Psychiatry. 2023. PMID: 37286536 Free PMC article. Clinical Trial.
-
REL-1017 (Esmethadone), A Novel NMDAR Blocker for the Treatment of MDD is Not Neurotoxic in Sprague-Dawley Rats.Front Pharmacol. 2022 Apr 25;13:863959. doi: 10.3389/fphar.2022.863959. eCollection 2022. Front Pharmacol. 2022. PMID: 35571103 Free PMC article.
-
Pharmacological Comparative Characterization of REL-1017 (Esmethadone-HCl) and Other NMDAR Channel Blockers in Human Heterodimeric N-Methyl-D-Aspartate Receptors.Pharmaceuticals (Basel). 2022 Aug 13;15(8):997. doi: 10.3390/ph15080997. Pharmaceuticals (Basel). 2022. PMID: 36015145 Free PMC article.
-
Esmethadone-HCl (REL-1017): a promising rapid antidepressant.Eur Arch Psychiatry Clin Neurosci. 2023 Oct;273(7):1463-1476. doi: 10.1007/s00406-023-01571-4. Epub 2023 Mar 8. Eur Arch Psychiatry Clin Neurosci. 2023. PMID: 36890259 Free PMC article. Review.
-
Psychosis after buprenorphine, heroin, methadone, morphine, oxycodone, and tramadol withdrawal: a systematic review.Eur Rev Med Pharmacol Sci. 2021 Jul;25(13):4554-4562. doi: 10.26355/eurrev_202107_26248. Eur Rev Med Pharmacol Sci. 2021. PMID: 34286498
Cited by
-
Letter to the Editor regarding 'Unique pharmacodynamic properties and low abuse liability of the µ-opioid receptor ligand (S)-methadone'.Mol Psychiatry. 2024 Dec;29(12):3935-3937. doi: 10.1038/s41380-024-02621-6. Epub 2024 May 28. Mol Psychiatry. 2024. PMID: 38806691 Free PMC article. No abstract available.
-
New Synthesis and Pharmacological Evaluation of Enantiomerically Pure (R)- and (S)-Methadone Metabolites as N-Methyl-d-aspartate Receptor Antagonists.J Med Chem. 2025 Mar 13;68(5):5455-5470. doi: 10.1021/acs.jmedchem.4c02605. Epub 2025 Feb 25. J Med Chem. 2025. PMID: 39999356 Free PMC article.
-
N-methyl-D-aspartate Receptors and Depression: Linking Psychopharmacology, Pathology and Physiology in a Unifying Hypothesis for the Epigenetic Code of Neural Plasticity.Pharmaceuticals (Basel). 2024 Nov 30;17(12):1618. doi: 10.3390/ph17121618. Pharmaceuticals (Basel). 2024. PMID: 39770460 Free PMC article. Review.
-
Reply to "Letter to the Editor regarding 'Unique pharmacodynamic properties and low abuse liability of the μ-opioid receptor ligand (S)-methadone'".Mol Psychiatry. 2025 Apr;30(4):1708-1709. doi: 10.1038/s41380-024-02864-3. Epub 2024 Dec 6. Mol Psychiatry. 2025. PMID: 39639176 No abstract available.
-
Drug-Drug Interaction Studies of Esmethadone (REL-1017) Involving CYP3A4- and CYP2D6-Mediated Metabolism.Drugs R D. 2024 Mar;24(1):51-68. doi: 10.1007/s40268-023-00450-6. Epub 2023 Nov 27. Drugs R D. 2024. PMID: 38010591 Free PMC article. Clinical Trial.
References
-
- Bernstein G, et al. Characterization of the safety and pharmacokinetic profile of D-methadone, a novel N-methyl-d-aspartate receptor antagonist in healthy, opioidnaive subjects: Results of two Phase 1 studies. J. Clin. Psychopharmacol. 2019;39:226–237. doi: 10.1097/JCP.0000000000001035. - DOI - PubMed

