Comparative analysis of human immune responses following SARS-CoV-2 vaccination with BNT162b2, mRNA-1273, or Ad26.COV2.S
- PMID: 35794181
- PMCID: PMC9258461
- DOI: 10.1038/s41541-022-00504-x
Comparative analysis of human immune responses following SARS-CoV-2 vaccination with BNT162b2, mRNA-1273, or Ad26.COV2.S
Abstract
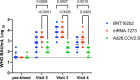
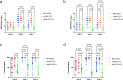
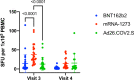
SARS-CoV-2 vaccines BNT162b2, mRNA-1273, and Ad26.COV2.S received emergency use authorization by the U.S. Food and Drug Administration in 2020/2021. Individuals being vaccinated were invited to participate in a prospective longitudinal comparative study of immune responses elicited by the three vaccines. In this observational cohort study, immune responses were evaluated using a SARS-CoV-2 spike protein receptor-binding domain ELISA, SARS-CoV-2 virus neutralization assays and an IFN- γ ELISPOT assay at various times over six months following initial vaccination. mRNA-based vaccines elicited higher magnitude humoral responses than Ad26.COV2.S; mRNA-1273 elicited the most durable humoral response, and all humoral responses waned over time. Neutralizing antibodies against the Delta variant were of lower magnitude than the wild-type strain for all three vaccines. mRNA-1273 initially elicited the greatest magnitude of T cell response, but this declined by 6 months. Declining immunity over time supports the use of booster dosing, especially in the setting of emerging variants.
© 2022. The Author(s).
Conflict of interest statement
J.M.M. and A.H. receive funding from NIH/NIAID (UM1AI148452) and are investigators for the adult mRNA1273 and Ad26.COV2.S phase 3 vaccine studies. J.V.W. serves on the Scientific Advisory Board of Quidel and an Independent Data Monitoring Committee for GlaxoSmithKline, neither related to the present work. All authors declare no competing non-financial interests.
Figures
Similar articles
-
Humoral and cellular immunogenicity of homologous and heterologous booster vaccination in Ad26.COV2.S-primed individuals: Comparison by breakthrough infection.Front Immunol. 2023 Mar 7;14:1131229. doi: 10.3389/fimmu.2023.1131229. eCollection 2023. Front Immunol. 2023. PMID: 36960070 Free PMC article.
-
Comparison of BNT162b2-, mRNA-1273- and Ad26.COV2.S-Elicited IgG and Neutralizing Titers against SARS-CoV-2 and Its Variants.Vaccines (Basel). 2022 May 27;10(6):858. doi: 10.3390/vaccines10060858. Vaccines (Basel). 2022. PMID: 35746466 Free PMC article.
-
Durability of Heterologous and Homologous COVID-19 Vaccine Boosts.JAMA Netw Open. 2022 Aug 1;5(8):e2226335. doi: 10.1001/jamanetworkopen.2022.26335. JAMA Netw Open. 2022. PMID: 35947380 Free PMC article.
-
Heterologous Ad26.COV2.S booster after primary BBIBP-CorV vaccination against SARS-CoV-2 infection: 1-year follow-up of a phase 1/2 open-label trial.Vaccine. 2024 Jul 25;42(19):3999-4010. doi: 10.1016/j.vaccine.2024.05.010. Epub 2024 May 13. Vaccine. 2024. PMID: 38744598 Clinical Trial.
-
Immunogenicity and clinical features relating to BNT162b2 messenger RNA COVID-19 vaccine, Ad26.COV2.S and ChAdOx1 adenoviral vector COVID-19 vaccines: a systematic review of non-interventional studies.Futur J Pharm Sci. 2022;8(1):20. doi: 10.1186/s43094-022-00409-5. Epub 2022 Mar 26. Futur J Pharm Sci. 2022. PMID: 35368622 Free PMC article. Review.
Cited by
-
Humoral Immune Response Diversity to Different COVID-19 Vaccines: Implications for the "Green Pass" Policy.Front Immunol. 2022 May 11;13:833085. doi: 10.3389/fimmu.2022.833085. eCollection 2022. Front Immunol. 2022. PMID: 35634315 Free PMC article.
-
Circulating Antibodies Against Common Cold Coronaviruses Do Not Interfere with Immune Responses to Primary or Booster SARS-CoV-2 mRNA Vaccines.Vaccines (Basel). 2025 May 21;13(5):547. doi: 10.3390/vaccines13050547. Vaccines (Basel). 2025. PMID: 40432156 Free PMC article.
-
Th1-dominant cytokine responses in kidney patients after COVID-19 vaccination are associated with poor humoral responses.NPJ Vaccines. 2023 May 17;8(1):70. doi: 10.1038/s41541-023-00664-4. NPJ Vaccines. 2023. PMID: 37198189 Free PMC article.
-
Maternal immune response and placental antibody transfer after COVID-19 vaccination across trimester and platforms.Nat Commun. 2022 Jun 28;13(1):3571. doi: 10.1038/s41467-022-31169-8. Nat Commun. 2022. PMID: 35764643 Free PMC article.
-
Ultrasound-Guided Fine Needle Biopsy of Human Axillary Lymph Nodes to Assess B Cell Responses to Vaccination.Methods Mol Biol. 2024;2826:15-30. doi: 10.1007/978-1-0716-3950-4_2. Methods Mol Biol. 2024. PMID: 39017882
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

