Immune tolerance of food is mediated by layers of CD4+ T cell dysfunction
- PMID: 35794484
- PMCID: PMC10336534
- DOI: 10.1038/s41586-022-04916-6
Immune tolerance of food is mediated by layers of CD4+ T cell dysfunction
Abstract
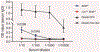
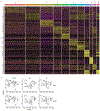
Gastrointestinal health depends on the adaptive immune system tolerating the foreign proteins in food1,2. This tolerance is paradoxical because the immune system normally attacks foreign substances by generating inflammation. Here we addressed this conundrum by using a sensitive cell enrichment method to show that polyclonal CD4+ T cells responded to food peptides, including a natural one from gliadin, by proliferating weakly in secondary lymphoid organs of the gut-liver axis owing to the action of regulatory T cells. A few food-specific T cells then differentiated into T follicular helper cells that promoted a weak antibody response. Most cells in the expanded population, however, lacked canonical T helper lineage markers and fell into five subsets dominated by naive-like or T follicular helper-like anergic cells with limited capacity to form inflammatory T helper 1 cells. Eventually, many of the T helper lineage-negative cells became regulatory T cells themselves through an interleukin-2-dependent mechanism. Our results indicate that exposure to food antigens causes cognate CD4+ naive T cells to form a complex set of noncanonical hyporesponsive T helper cell subsets that lack the inflammatory functions needed to cause gut pathology and yet have the potential to produce regulatory T cells that may suppress it.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
COMPETING INTERESTS
The authors declare no competing interests.
Figures
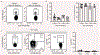






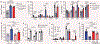
References
-
- Renz H et al. Food allergy. Nat Rev Dis Primers 4, 17098 (2018). - PubMed
-
- Green PH, Lebwohl B & Greywoode R Celiac disease. J Allergy Clin Immunol 135, 1099–1106; quiz 1107 (2015). - PubMed
-
- Mowat AM To respond or not to respond - a personal perspective of intestinal tolerance. Nat Rev Immunol 18, 405–415 (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

