SARS-CoV-2 Infection During Pregnancy—An Analysis of Clinical Data From Germany and Austria From the CRONOS Registry
- PMID: 35794736
- PMCID: PMC9749842
- DOI: 10.3238/arztebl.m2022.0266
SARS-CoV-2 Infection During Pregnancy—An Analysis of Clinical Data From Germany and Austria From the CRONOS Registry
Abstract
Background: Using data from the German CRONOS registry, we assessed the risk of a complicated course of COVID-19 in women with a SARS-CoV-2-infection during pregnancy, with particular consideration of gestational age, vaccination status, and pandemic dynamics.
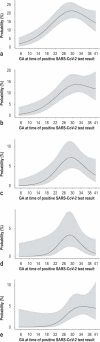
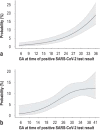
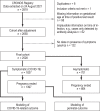
Methods: Data acquired in two separate periods (March 2020 to August 2021; January to June 2022) for CRONOS, a prospective, hospital-based observational study (DRKS00021208), were studied with logistic regression models. Odds ratios comparing 32 with 22 weeks of gestation were calculated for relevant COVID-19-specific events occurring within 4 weeks of a positive test result.
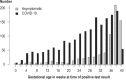
Results: Data from 3481 women were evaluated. The risk of all of the defined COVID-19-specific events was low among women who became ill with COVID-19 during the first trimester and rose with increasing gestational age into the early third trimester. For example, the odds ratio for hospitalization because of a COVID-19 infection, comparing 32 versus 22 weeks of gestation, was 1.4 (95% confidence interval [1.2; 1.7]). This risk was lower in the second period of data acquisition than in the first (OR 0.66; 95% CI [0.50; 0.88]), and it was even lower if the pregnant patient had been vaccinated against COVID-19 (OR 0.27; 95% CI [0.18; 0.41]).
Conclusion: These findings can serve as a basis for counseling about prophylactic or therapeutic measures, such as the administration of monoclonal antibodies. They underscore the efficacy of vaccination for pregnant women even during the omicron phase of the pandemic.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

