Alterations in Inflammatory Cytokines and Redox Homeostasis in LPS-Induced Pancreatic Beta-Cell Toxicity and Mitochondrial Stress: Protection by Azadirachtin
- PMID: 35794865
- PMCID: PMC9251516
- DOI: 10.3389/fcell.2022.867608
Alterations in Inflammatory Cytokines and Redox Homeostasis in LPS-Induced Pancreatic Beta-Cell Toxicity and Mitochondrial Stress: Protection by Azadirachtin
Abstract
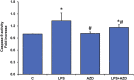
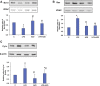
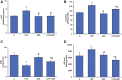
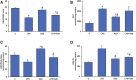
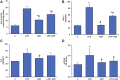
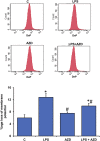
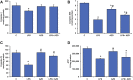
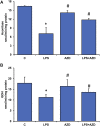
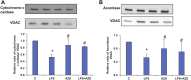
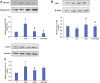
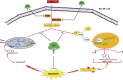
Inflammation and redox imbalance are hallmarks of cancer, diabetes, and other degenerative disorders. Pathophysiological response to these disorders leads to oxidative stress and mitochondrial dysfunction by alterations and reprogramming in cellular signaling and metabolism. Pancreatic beta cells are very sensitive to the inflammatory and altered nutrient signals and hence play a crucial role in diabetes and cancer. In this study, we treated insulin-secreting pancreatic beta cells, Rin-5F, with the bacterial endotoxin, LPS (1 μg/ml) to induce an inflammatory response in vitro and then treated the cells with a known anti-inflammatory, anticancer and antioxidant phytochemical, azadirachtin (AZD, 25 µM for 24 h). Our results demonstrated lipid peroxidation and nitric oxide production causing increased nitro/oxidative stress and alterations in the activities of anti-oxidant enzymes, superoxide dismutase and catalase after LPS treatment. Pro-inflammatory responses caused by translocation of nuclear factor kappa B and release of inflammatory cytokines were also observed. These changes were accompanied by GSH-dependent redox imbalance and alterations in mitochondrial membrane potential and respiratory complexes enzyme activities leading to mitochondrial respiratory dysfunction, reduced ATP synthesis, and intrinsic caspase-9 mediated apoptosis. Caspase-9 was activated due to alterations in Bcl-2 and Bax proteins and release of cytochrome c into the cytosol. The activities of oxidative stress-sensitive mitochondrial matrix enzymes, aconitase, and glutamate dehydrogenase were also inhibited. Treatment with AZD showed beneficial effects on the recovery of antioxidant enzymes, inflammatory responses, and mitochondrial functions. GSH-dependent redox homeostasis also recovered after the treatment with AZD. This study may help in better understanding the etiology and pathogenesis of inflammation-induced disorders in pancreatic beta cells to better manage therapeutic strategies.
Keywords: GSH redox metabolism; LPS; azadirachtin; inflammation signaling; mitochondria; pancreatic cell.
Copyright © 2022 John and Raza.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Al-Nahdi A. M. T., John A., Raza H. (2018). Cytoprotective Effects of N-Acetylcysteine on Streptozotocin- Induced Oxidative Stress and Apoptosis in RIN-5F Pancreatic β-Cells. Cell. Physiol. biochem. 51, 201–216. 10.1159/000495200 PubMed Abstract | 10.1159/000495200 | Google Scholar - DOI - DOI - PubMed
-
- Alnahdi A., John A., Raza H. (2019a). Augmentation of Glucotoxicity, Oxidative Stress, Apoptosis and Mitochondrial Dysfunction in HepG2 Cells by Palmitic Acid. Nutrients 11, 1979. 10.3390/nu11091979 PubMed Abstract | 10.3390/nu11091979 | Google Scholar - DOI - DOI - PMC - PubMed
-
- Alnahdi A., John A., Raza H. (2020). Mitigation of Glucolipotoxicity-Induced Apoptosis, Mitochondrial Dysfunction, and Metabolic Stress by N-Acetyl Cysteine in Pancreatic β-Cells. Biomolecules 10, 239. 10.3390/biom10020239 PubMed Abstract | 10.3390/biom10020239 | Google Scholar - DOI - DOI - PMC - PubMed
-
- Alnahdi A., John A., Raza H. (2019b). N-acetyl Cysteine Attenuates Oxidative Stress and Glutathione-dependent Redox Imbalance Caused by High Glucose/high Palmitic Acid Treatment in Pancreatic Rin-5F Cells. PLoS ONE 14, e0226696. 10.1371/journal.pone.0226696 PubMed Abstract | 10.1371/journal.pone.0226696 | Google Scholar - DOI - DOI - PMC - PubMed
-
- Amyot J., Semache M., Ferdaoussi M., Fontés G., Poitout V. (2012). Lipopolysaccharides Impair Insulin Gene Expression in Isolated Islets of Langerhans via Toll-like Receptor-4 and NF-Κb Signalling. PLOS ONE 7, e36200. 10.1371/journal.pone.0036200 PubMed Abstract | 10.1371/journal.pone.0036200 | Google Scholar - DOI - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

