Mechanisms of group B Streptococcus-mediated preterm birth: lessons learnt from animal models
- PMID: 35794927
- PMCID: PMC9254271
- DOI: 10.1530/RAF-21-0105
Mechanisms of group B Streptococcus-mediated preterm birth: lessons learnt from animal models
Abstract
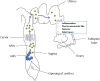
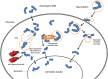
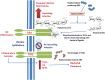
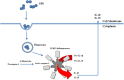
Group B Streptococcus (GBS) is an opportunistic pathogenic bacterium which upon colonization in the female reproductive tract can cause preterm births, fetal injury, and demise. Several determinants for GBS pathogenesis have been explored so far through the studies using animal models ranging from mice to non-human primates. The results from these experimental data have identified outer membrane vesicles, β-hemolysin, hyaluronidase, and Cas9 of GBS as major virulence factors leading to preterm births. Most of these factors drive inflammation through activation of NLRP3 and elevated production of IL1-β. However, the absence of one of the factors from the pathogen reduces but does not completely abolish the pathogenesis of GBS suggesting the involvement of more than one factor in causing preterm birth. This makes further exploration of other virulence factors of GBS pathogenesis important in gaining an insight into the mechanistic basis of GBS-mediated preterm births.
Lay summary: Group B Streptococcus (GBS) is a pathogenic bacteria whose infection in the reproductive tract during pregnancy can cause premature delivery. This bacterial infection is one of the major causes of death of mother and baby during pregnancy, and the bacteria is prevalent in all parts of the world. This makes the research on GBS so important and many of the mechanisms behind GBS infection during pregnancy still remain unexplored. In this review, we have outlined how various animal models contributed in finding the mechanism of GBS pathogenesis. The review also focuses on compiling various virulence factors which makes GBS pathogenic in the vulnerable. Understanding the mechanisms of infection by GBS will be crucial in developing drugs and vaccines to protect against the harmful effects of the bacteria.
Keywords: Streptococcus agalactiae; animal model; infection; membrane rupture; pathogenesis; pregnancy.
© The authors.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

