PK-modifying anchors significantly alter clearance kinetics, tissue distribution, and efficacy of therapeutics siRNAs
- PMID: 35795486
- PMCID: PMC9240963
- DOI: 10.1016/j.omtn.2022.06.005
PK-modifying anchors significantly alter clearance kinetics, tissue distribution, and efficacy of therapeutics siRNAs
Abstract
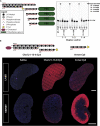
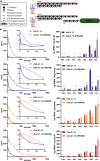
Effective systemic delivery of small interfering RNAs (siRNAs) to tissues other than liver remains a challenge. siRNAs are small (∼15 kDa) and therefore rapidly cleared by the kidneys, resulting in limited blood residence times and tissue exposure. Current strategies to improve the unfavorable pharmacokinetic (PK) properties of siRNAs rely on enhancing binding to serum proteins through extensive phosphorothioate modifications or by conjugation of targeting ligands. Here, we describe an alternative strategy for enhancing blood and tissue PK based on dynamic modulation of the overall size of the siRNA. We engineered a high-affinity universal oligonucleotide anchor conjugated to a high-molecular-weight moiety, which binds to the 3' end of the guide strand of an asymmetric siRNA. Data showed a strong correlation between the size of the PK-modifying anchor and clearance kinetics. Large 40-kDa PK-modifying anchors reduced renal clearance by ∼23-fold and improved tissue exposure area under the curve (AUC) by ∼26-fold, resulting in increased extrahepatic tissue retention (∼3- to 5-fold). Furthermore, PK-modifying oligonucleotide anchors allowed for straightforward and versatile modulation of blood residence times and biodistribution of a panel of chemically distinct ligands. The effects were more pronounced for conjugates with low lipophilicity (e.g., N-Acetylgalactosamine [GalNAc]), where significant improvement in uptake by hepatocytes and dose-dependent silencing in the liver was observed.
Keywords: GalNAc; Gene silencing; PEGylation; RNA interference; extrahepatic; oligonucleotide; siRNA conjugates.
© 2022 The Authors.
Conflict of interest statement
B.M.D.C.G., M.R.H., and A.K. have filed a patent application for dynamic PK-modifying oligonucleotide anchors.
Figures





References
-
- Godinho B.M., Khvorova A. The era of RNA interference medicines: the clinical landscape of synthetic gene silencing drugs. Saúde Tecnol. 2019;21:05–17.
-
- Adams D., Gonzalez-Duarte A., O’Riordan W.D., Yang C.-C., Ueda M., Kristen A.V., Tournev I., Schmidt H.H., Coelho T., Berk J.L., et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N. Engl. J. Med. 2018;379:11–21. - PubMed
-
- Balwani M., Sardh E., Ventura P., Peiró P.A., Rees D.C., Stölzel U., Bissell D.M., Bonkovsky H.L., Windyga J., Anderson K.E., et al. Phase 3 trial of RNAi therapeutic givosiran for acute intermittent porphyria. N. Engl. J. Med. 2020;382:2289–2301. - PubMed
-
- Morrissey D.V., Lockridge J.A., Shaw L., Blanchard K., Jensen K., Breen W., Hartsough K., Machemer L., Radka S., Jadhav V., et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat. Biotechnol. 2005;23:1002–1007. - PubMed
-
- Christensen J., Litherland K., Faller T., Van de Kerkhof E., Natt F., Hunziker J., Krauser J., Swart P. Metabolism studies of unformulated internally [3H]-labeled short interfering RNAs in mice. Drug Metab. Dispos. 2013;41:1211–1219. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

