Regulation of HCN Channels by Protein Interactions
- PMID: 35795651
- PMCID: PMC9251338
- DOI: 10.3389/fphys.2022.928507
Regulation of HCN Channels by Protein Interactions
Abstract
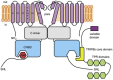
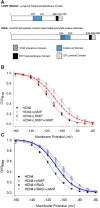
Hyperpolarization-activated, cyclic nucleotide-sensitive (HCN) channels are key regulators of subthreshold membrane potentials in excitable cells. The four mammalian HCN channel isoforms, HCN1-HCN4, are expressed throughout the body, where they contribute to diverse physiological processes including cardiac pacemaking, sleep-wakefulness cycles, memory, and somatic sensation. While all HCN channel isoforms produce currents when expressed by themselves, an emerging list of interacting proteins shape HCN channel excitability to influence the physiologically relevant output. The best studied of these regulatory proteins is the auxiliary subunit, TRIP8b, which binds to multiple sites in the C-terminus of the HCN channels to regulate expression and disrupt cAMP binding to fine-tune neuronal HCN channel excitability. Less is known about the mechanisms of action of other HCN channel interaction partners like filamin A, Src tyrosine kinase, and MinK-related peptides, which have a range of effects on HCN channel gating and expression. More recently, the inositol trisphosphate receptor-associated cGMP-kinase substrates IRAG1 and LRMP (also known as IRAG2), were discovered as specific regulators of the HCN4 isoform. This review summarizes the known protein interaction partners of HCN channels and their mechanisms of action and identifies gaps in our knowledge.
Keywords: HCN channels; IRAG; KCNE; TRIP8b; accessory proteins; protein subunits.
Copyright © 2022 Peters, Singh, Bankston and Proenza.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Bankston J. R., Camp S. S., DiMaio F., Lewis A. S., Chetkovich D. M., Zagotta W. N. (2012). Structure and Stoichiometry of an Accessory Subunit TRIP8b Interaction with Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels. Proc. Natl. Acad. Sci. U.S.A. 109, 7899–7904. 10.1073/pnas.1201997109 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

